Which one of the following reacts faster in an SN1 reaction and why?
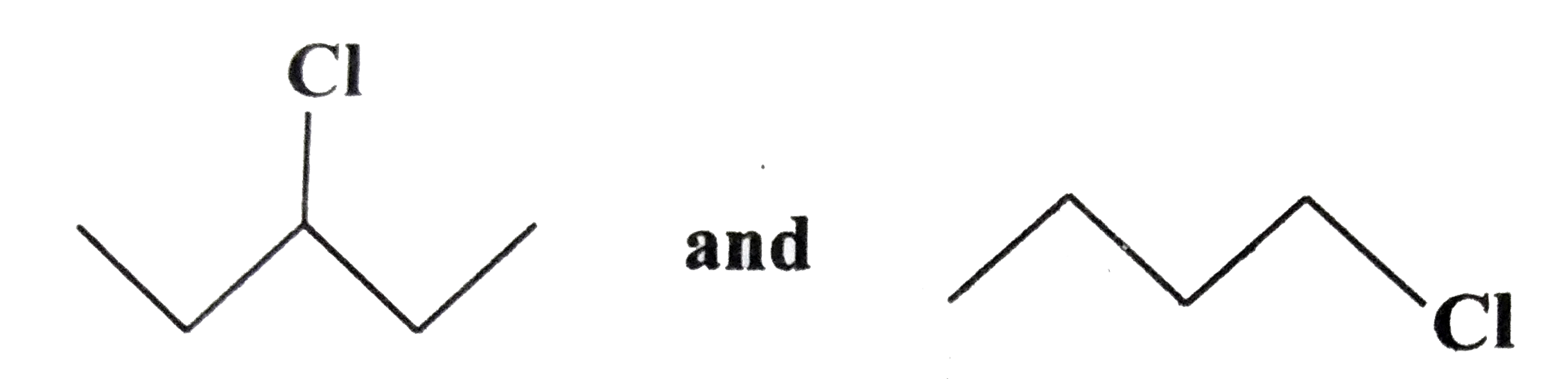
The reactivity is SN1 reactions depends upon the stability of the intermediate carbocation which ann alkyl halide gives on ionization. Thus.
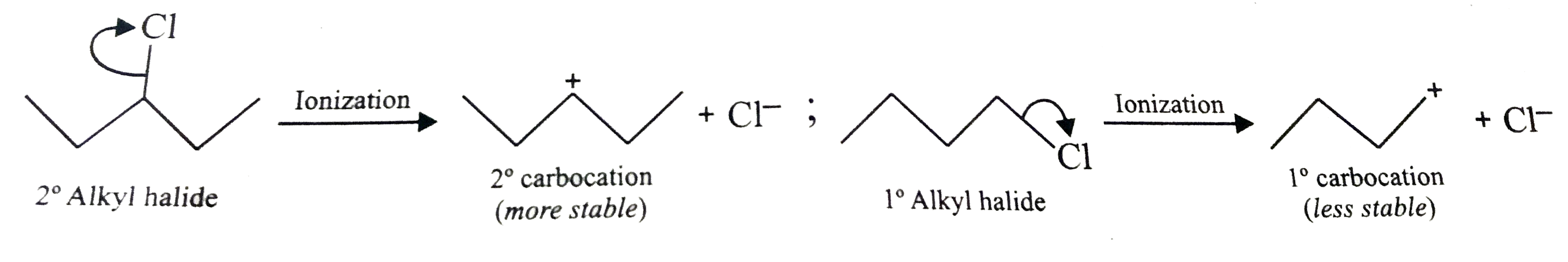
Since 2∘ carbocations is more stable than 1∘ carbocation, therefore 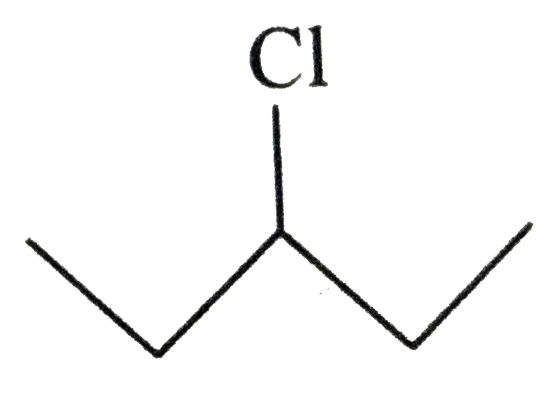 will react faster than
will react faster than 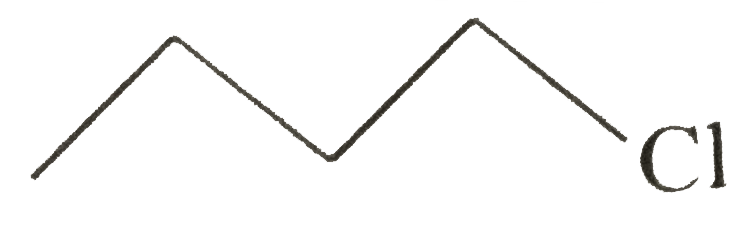 in SN1 reaction.
in SN1 reaction.
Similar Questions
Knowledge Check
Which one of the following will be most recctive for SN1 reaction?
A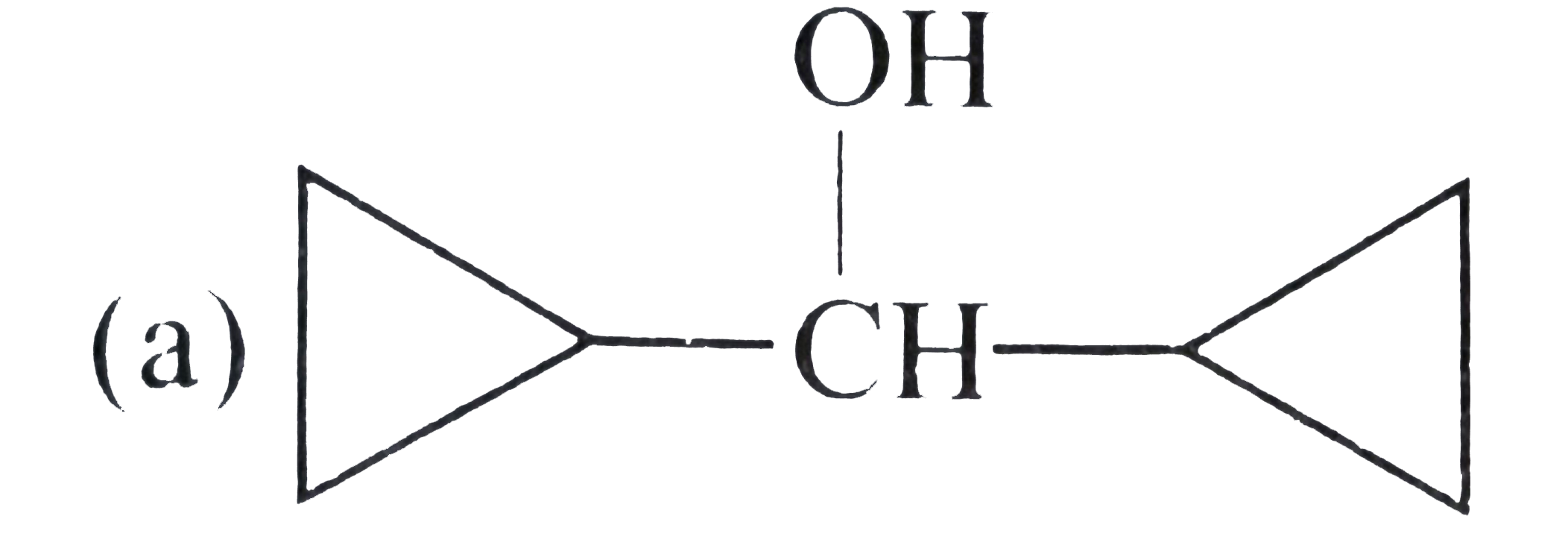 BC6H5−OH∣CH−CH6H5CCH3−OH∣C∣CH3−CH3D
BC6H5−OH∣CH−CH6H5CCH3−OH∣C∣CH3−CH3D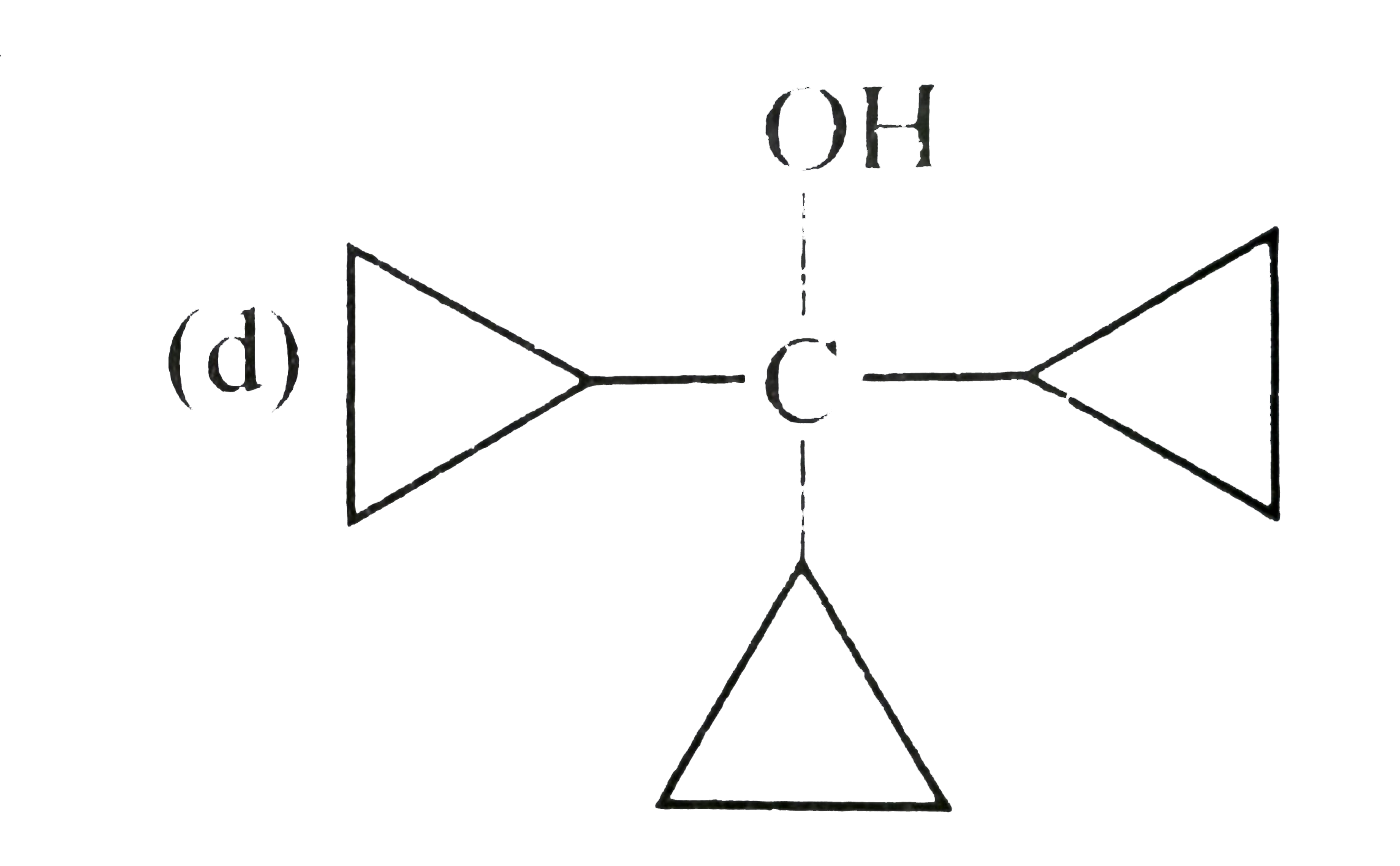
Which compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with OH−?
(a) CH3Br or CH3I
(b) CH2=CHBr or CH2=CHCH2Br.
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with −OH and why?
(i)CH3Br or CH3I (ii)(CH3)3CCl or CH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with -OH ? Why?
(i) CH3BrorCH3I (ii) (CH3)3CClorCH3Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with −OH :
(i) CH2Br or CH3I (ii) (CH3)3CCI or CH3CI ?
View SolutionWhich of the compounds will react faster in SN1 reaction with −OH ion?
CH3−CH2−ClorC6H5−CH2−Cl
View Solution

