The decreasing order of nucleophilicity among the nucleophiles
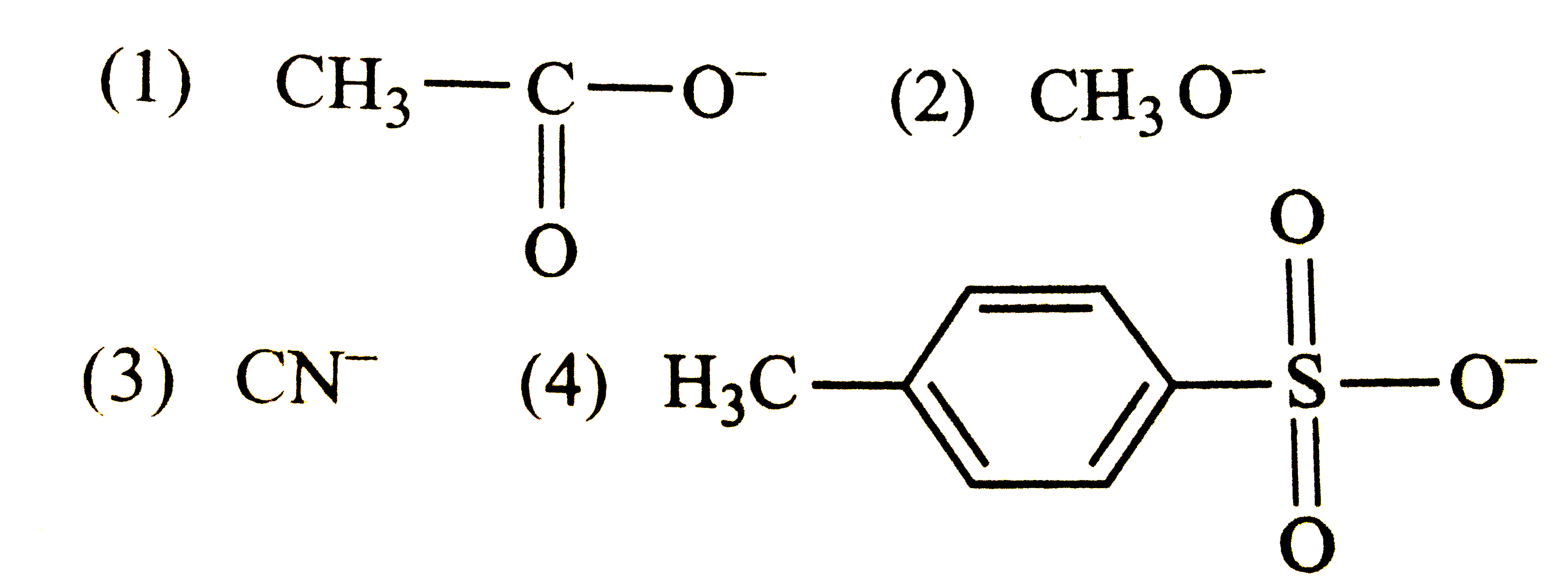 is
is
A
(1),(2),(3),(4)
B
(4),(3),(2),(1)
C
(2),(3),(1),(4)
D
(3,(2),(1),(4)
Video Solution
Text Solution
The correct Answer is:D
Because of lower electronegativity of C over O, CN− is a better nucleophile than methoxide ion, among acetate ion and p-toluenesulphonate ion, because of smaller resonance stabilization, CH3COO− is a better nucleophile than p-toluene-sulphonate ion.
|
Similar Questions
Knowledge Check
The decreasing order of nucleophilicity among the nucleophiles is :
(I) CH3−C∣∣O−O−
(II)CH3O−
(III) CN− (IV)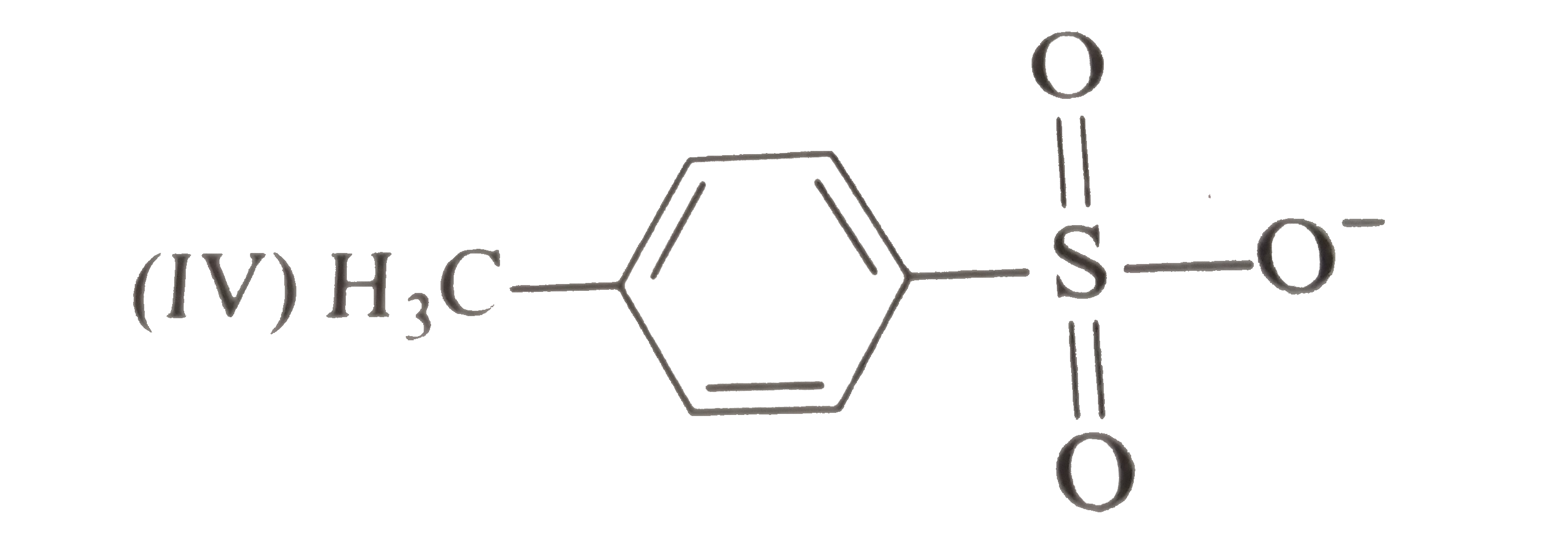 A(C),(B),(A),(D)B(B),(C),(A),(D)C(D),(C),(B),(A)D(A),(B),(C),(D)
A(C),(B),(A),(D)B(B),(C),(A),(D)C(D),(C),(B),(A)D(A),(B),(C),(D)The increasing order of nucleophilicity is:
AI−<Cl−<Br−BBr−<Cl−<I−CCl−<Br−<I−DI−<Br−<Cl−The correct order of nucleophilicity is
AI−>Br−>Cl−>F−BI−>Cl−>Br−>F−CF−>Cl−>Br−>I−DCl−>Br−>F−>I−
