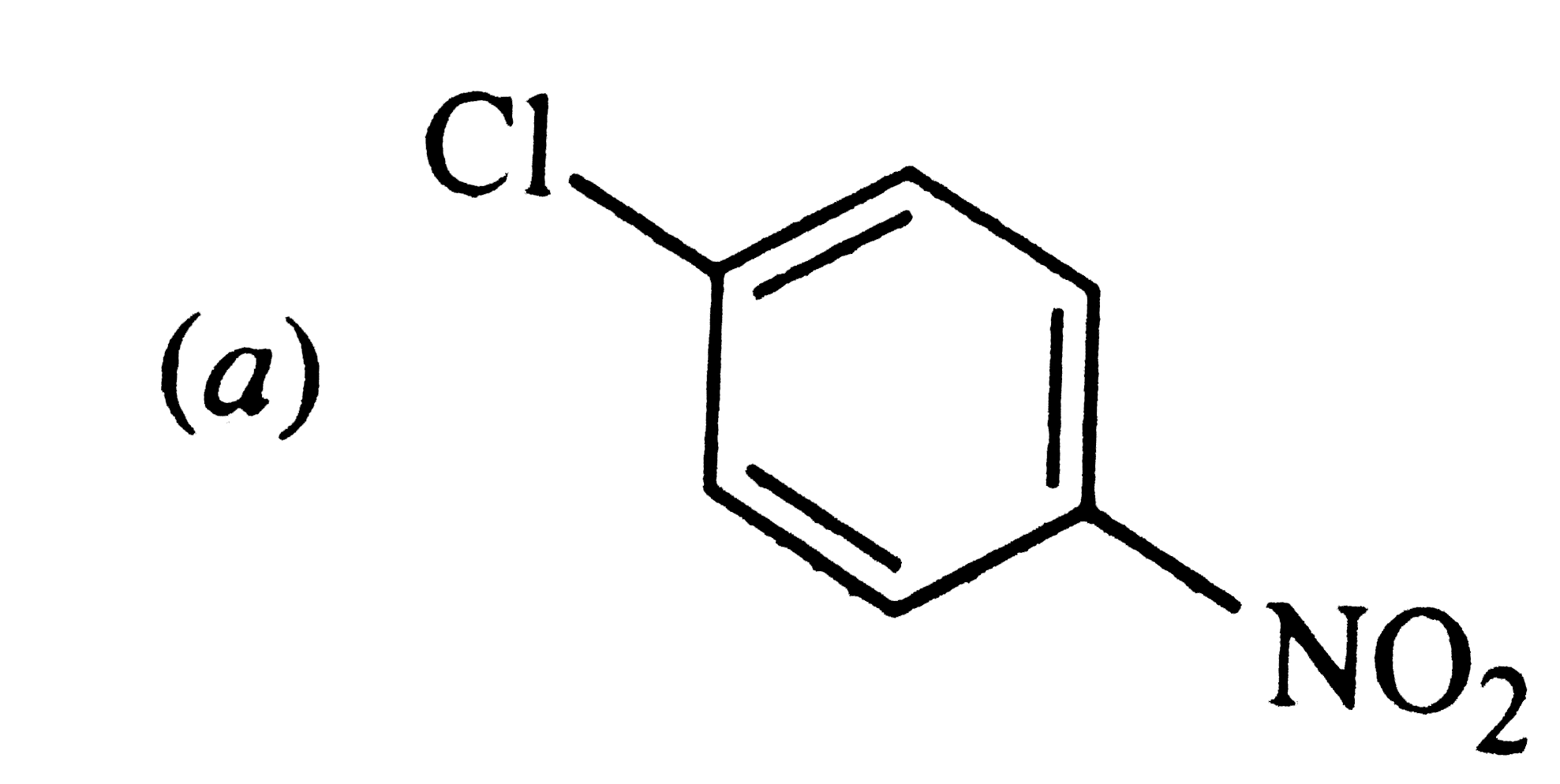
Which of the following compounds undergoes mucleophilic substitution reaction most easily?
A
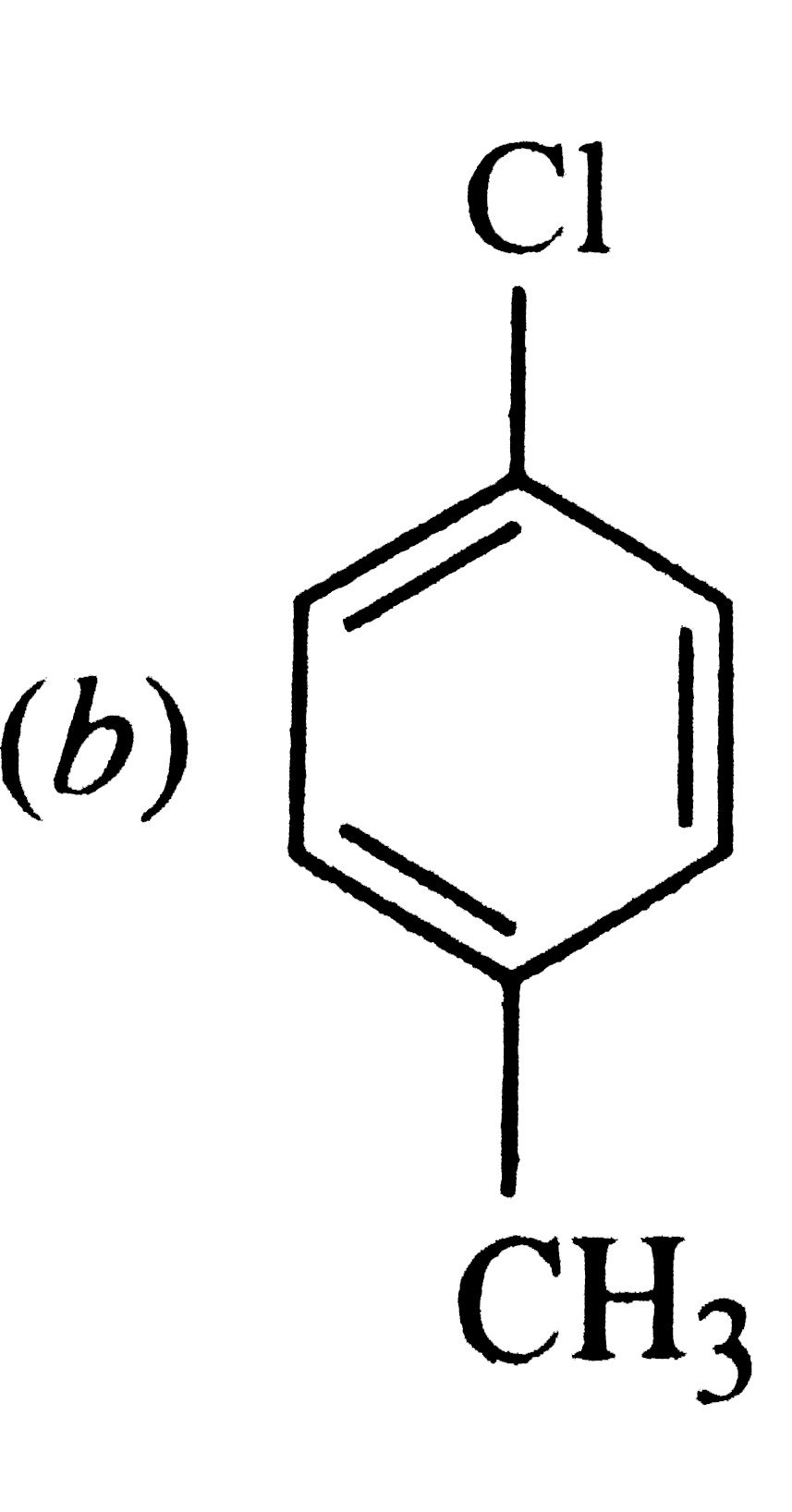
B
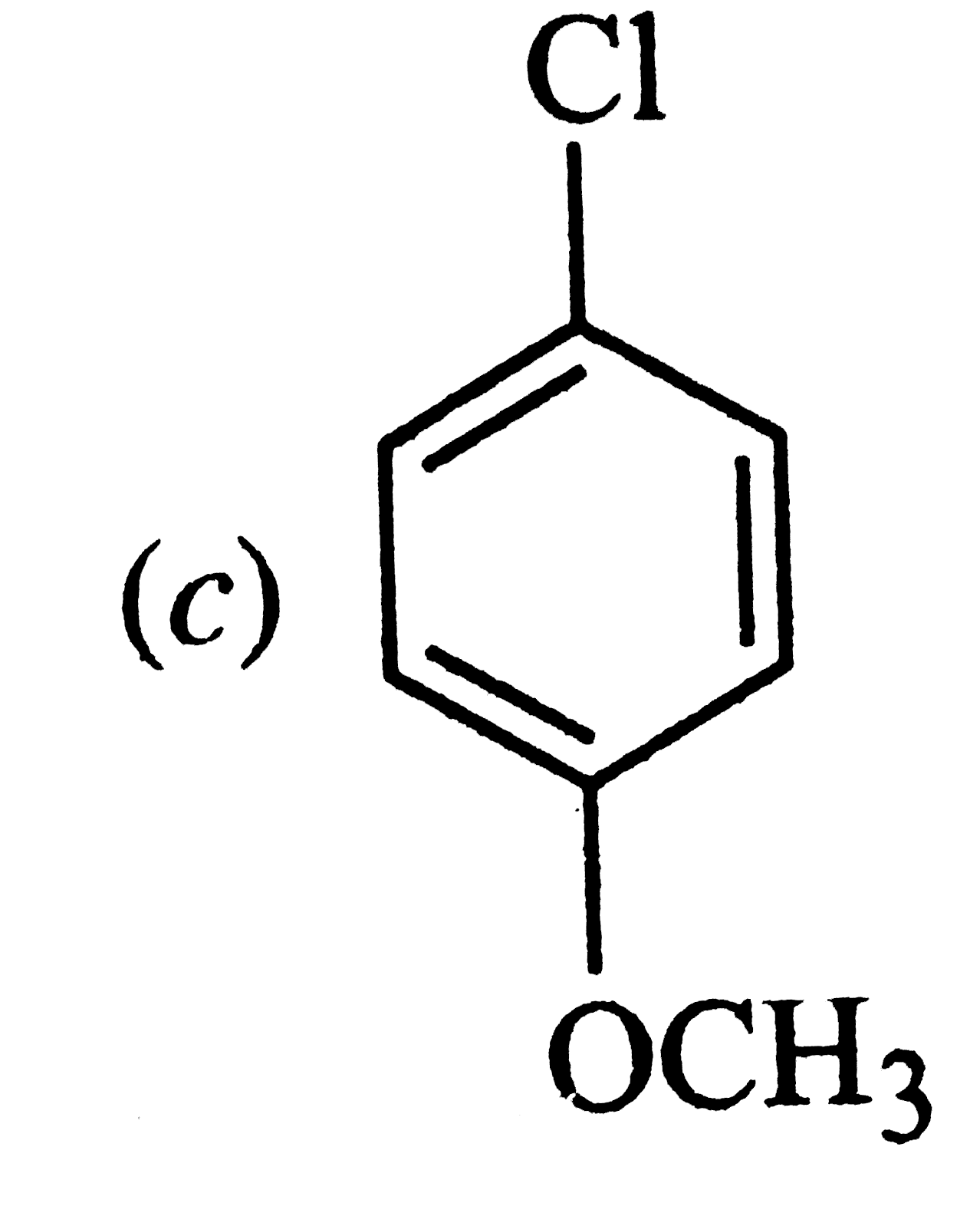
C
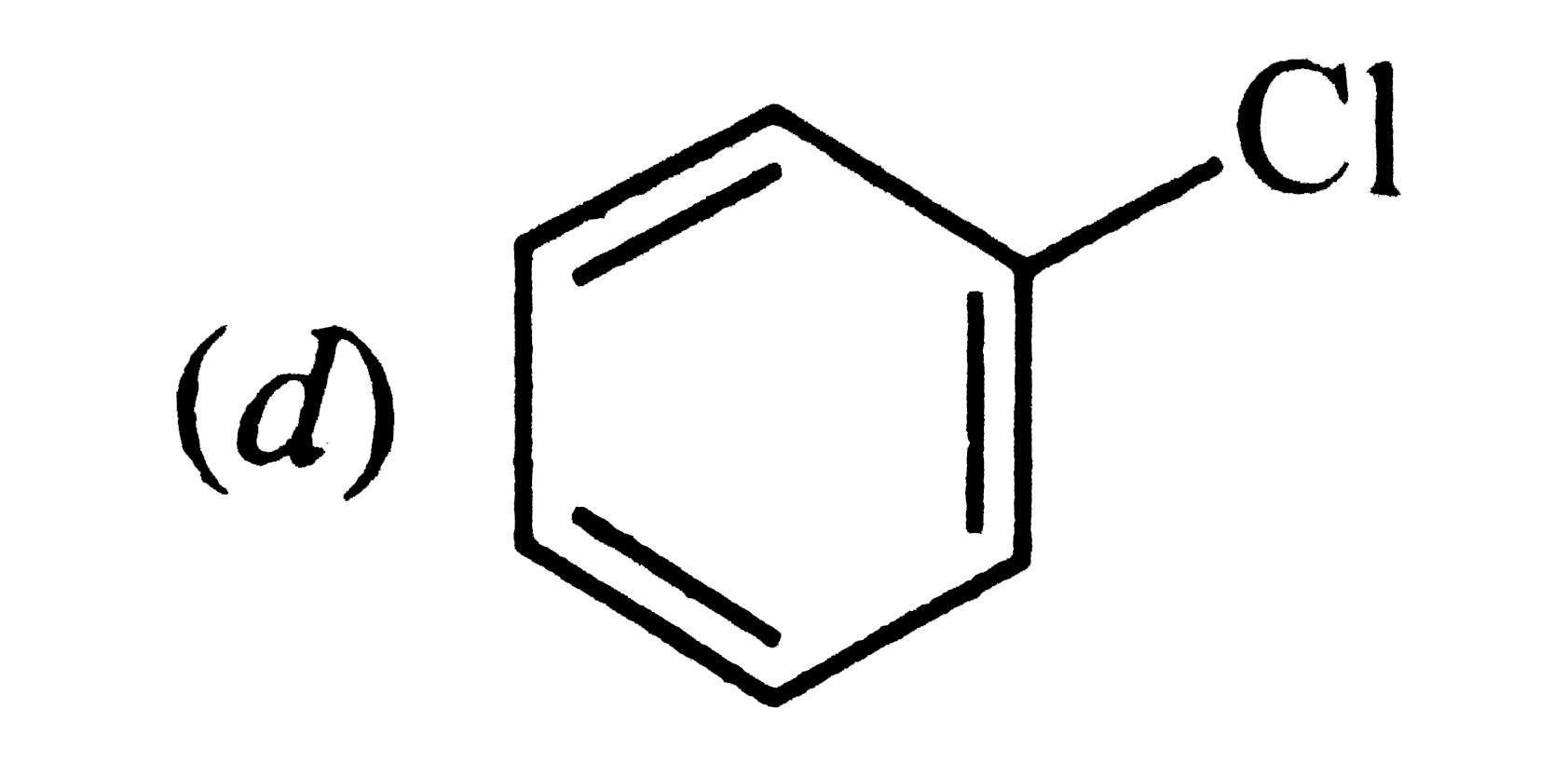
D
Video Solution
Text Solution
The correct Answer is:A
Presence of electron-withdrawing groups (i.e., NO2,CN, etc.) at o- and p-position w.r.t. Cl activates it towards nucleophilic substitution reactions while presence of electron-donating groups (i.e., CH3,OCH3, etc.) deactivates it towards nucleophilic substitution reaction. thus, p-nitrochlorobenzene undergoes nucleophilic substitution reaction most easily. thus, option (a) is correct.
|
Similar Questions
Knowledge Check
Which of the following compounds undergoes mucleophilic substitution reaction most easily?
A B
B C
C D
D
Which the following compound undergoes nucleophilic substitution reaction most easily
A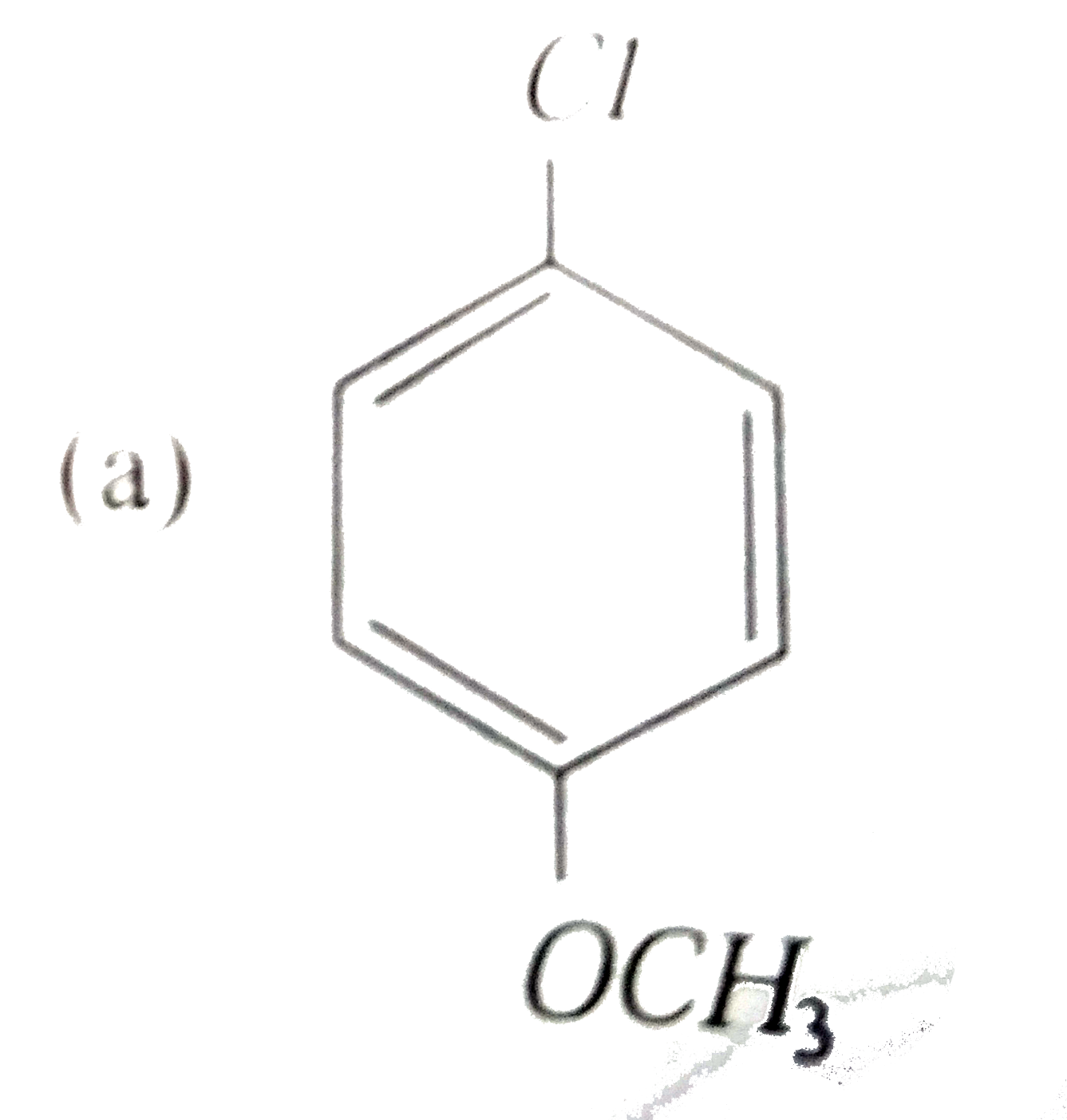 B
B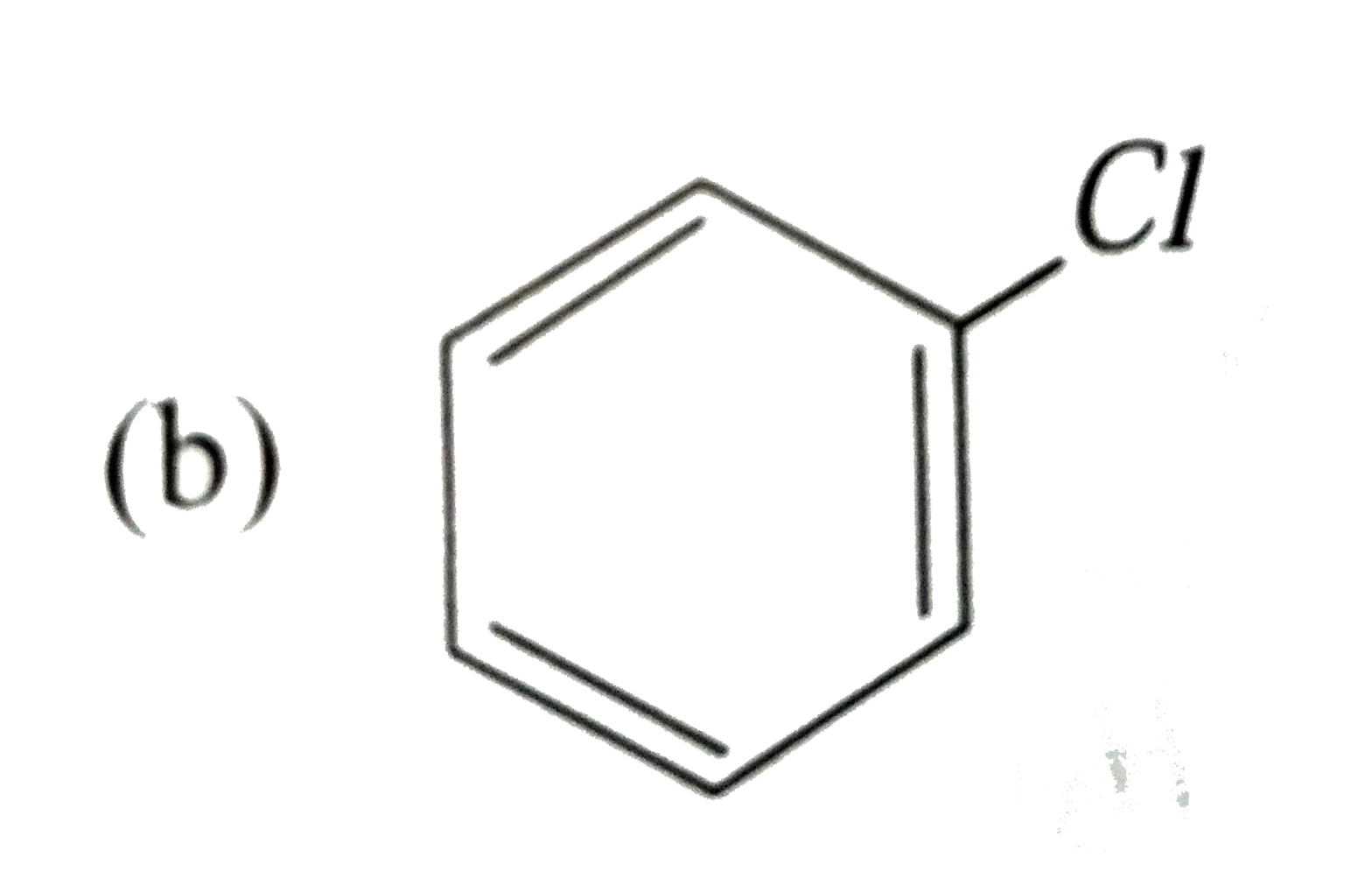 C
C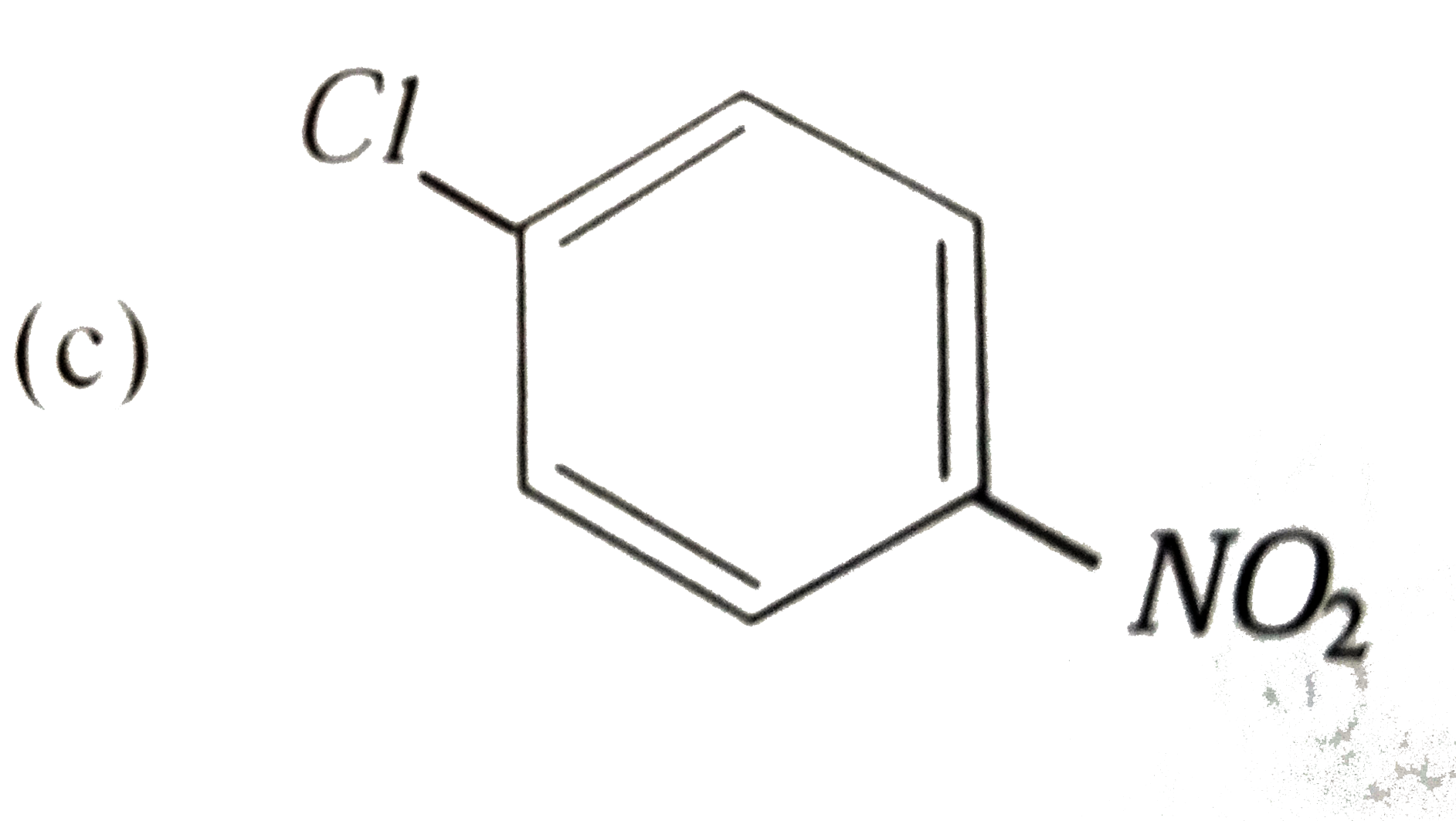 D
D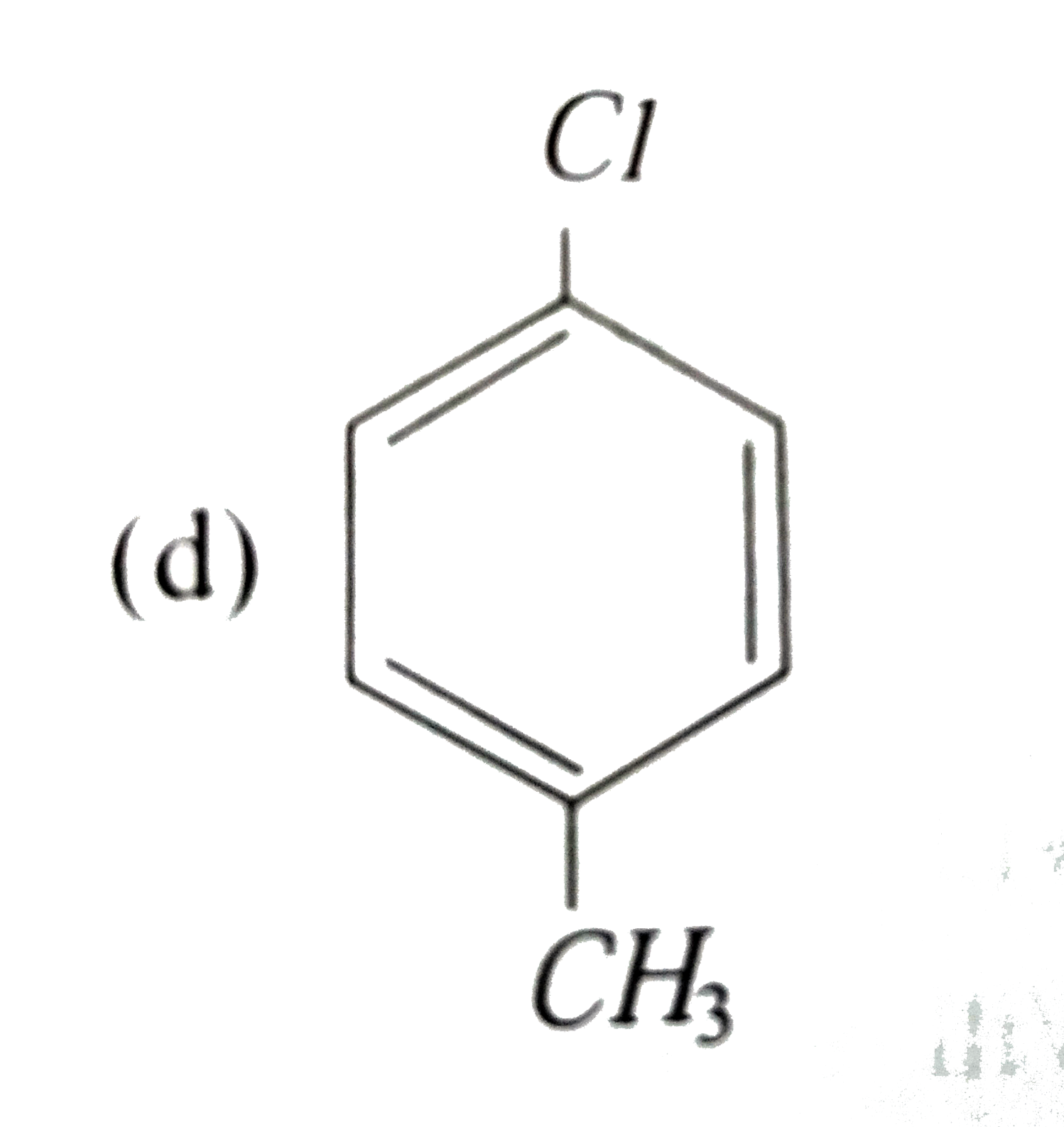
Which of the following compounds undergoes nucleophilic substance reaction most easily?
A B
B C
C D
D
Which of the following compounds undergoes
nucleophilic substitution most readly?
View SolutionWhich of the following compound will not undergo nucleophilic substitution reaction ?
View SolutionWhich of the following compounds does not undergo nucleophilic substitution reactions ?
View SolutionWhich of the following undergoes electrophilic substitution reactions faster than benzene?
View SolutionWhich of the following undergoes electrophilic substitution reactions faster than benzene?
View Solution

