Ethers behave as weakly______substances due to the presence of two lone pairs of electrons on the oxygen atom.
Step-by-Step Text Solution:
1. Understanding Ethers: Ethers are organic compounds that contain an oxygen atom connected to two alkyl or aryl groups. The general formula for ethers can be represented as R-O-R', where R and R' are hydrocarbon chains.
2. Lone Pairs on Oxygen: The oxygen atom in ethers has two lone pairs of electrons. These lone pairs play a crucial role in determining the chemical properties of ethers.
3. Basicity of Ethers: Due to the presence of these two lone pairs, ethers can act as weak bases. This is because the lone pairs can accept protons (H⁺ ions) from acids, which is a characteristic behavior of basic substances.
4. Electronegativity of Oxygen: Oxygen is a highly electronegative element. This means it has a strong tendency to attract electrons towards itself. As a result, while ethers can act as bases, they do so weakly because the electronegativity of oxygen makes it less likely to donate its lone pairs compared to other stronger bases.
5. Conclusion: Therefore, we can conclude that ethers behave as weakly basic substances due to the presence of two lone pairs of electrons on the oxygen atom.
Final Answer: Ethers behave as weakly basic substances due to the presence of two lone pairs of electrons on the oxygen atom.
---
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:CONCEPTUAL QUESTIONS
Explore 18 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT SOLVED QUESTIONS)
Explore 7 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:TEST YOUR GRIP (I. MULTIPLE CHOICE QUESTIONS)
Explore 19 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:PRADEEPChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 29 Videos
Similar Questions
An organic (A) reacts with PCl5 to produce another compound (B). (B) reacts with magnesium metal in presence of ether to produce a Grignard reagent (C). (C) reacts with ethanal and the3 product is hydrolysed to produce propan-2-ol. Identify (A), (B) and (C) and explaiin the reactions.
View SolutionGive the IUPAC names of the following compounds:
(i) CH3−C∣ClH−C∣CH3H−C∣CH3H−CH2OH
(ii) CH3−C∣CH3H−OCH2CH3

View SolutionThe general molecular formula, which represents the homologous series of alkanols is
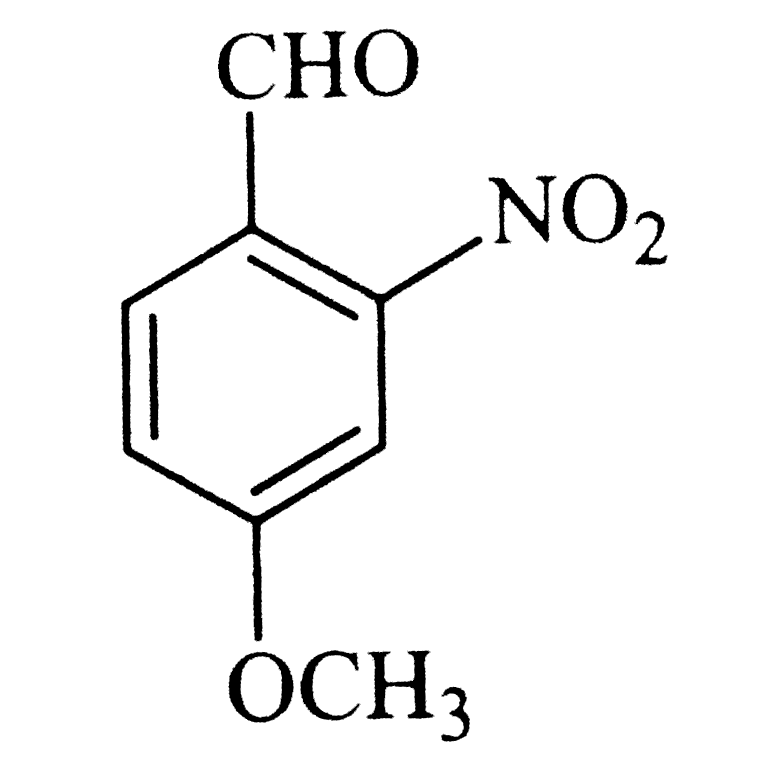
View SolutionGive the IUPAC name of the following compound
View SolutionAmmonia acts as a Lewis base due to the presence of lone pair of electrons on N-atom.
View SolutionAssertion : Ethers behave as bases in the presence of mineral acids.
Reason : Due to the presence of lone electrons pair on the oxygen atom.
View SolutionAssertion : Ethers behave as bases in the presence of mineral
Reason : Due to the presence of lone electron pair on the oxygen atom.
View Solution(A) Ether behaves as bases in the presence of mineral acids. (R ) Due to the presence of lone pair of electrons on oxygen.
View SolutionThe number of bond pair and lone pair on oxygen atom in ether are respectively
View SolutionThe compound with two lone pairs of electrons on the central atom is (are)
View SolutionA lone pair of electrons in an atom implies
View Solutionएक परमाणु में एक एकाकी इलेक्ट्रॉन युग्म प्रदर्शित करता है
View Solution(A): Ethers behave as bases in the presence of mineral acids.
(R): Oxygen atom in ether is having lone pair electrons.
View SolutionA: Ethers behave as bases in the presence of mineral acids. R: It is due to the presence of lone pair of electrons on the oxygen.
View Solutionकथनः ईथर मिनरल एसिड की उपस्थिति में क्षार की तरह व्यवहार करता है।
कारणः ईथर में ऑक्सीजन पर इलेक्ट्रॉन यग्म उपस्थित होता है।
View Solution___________ has two lone pairs of electrons on the central atom.
View Solution
