Anisole is less reactive than phenol towards electrophilic substitution reactions. Justify your answer with proper reasoning.
Video Solution
Text Solution
Both -OH and −OCH3 groups have +R-effect as shown below:
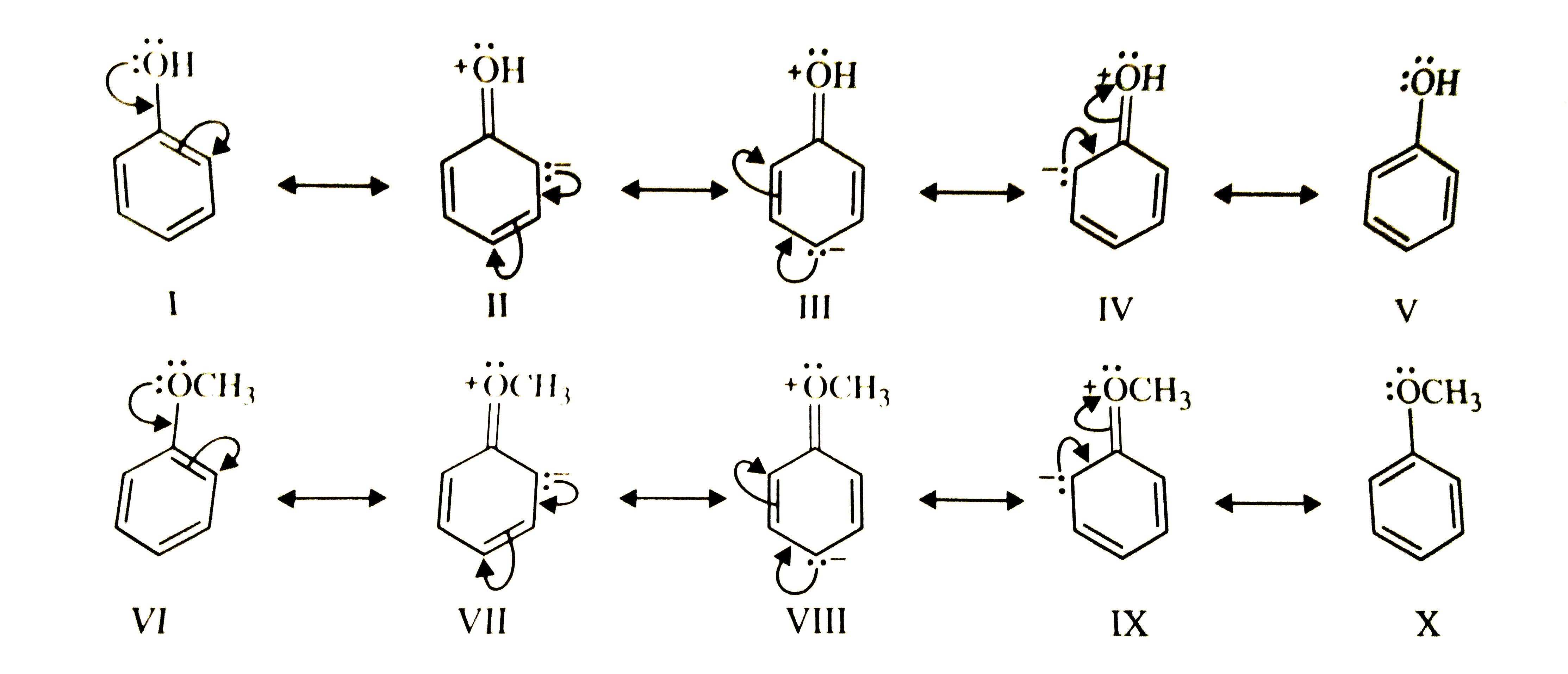
Structures (II to IV) involve separation of +ve and -ve charges. Similarly, structures (VII-IX) involve separation of +ve and -ve charges. But structures (II to IV) can stabilize themselves by releasing a proton which is a very stable chemical species.
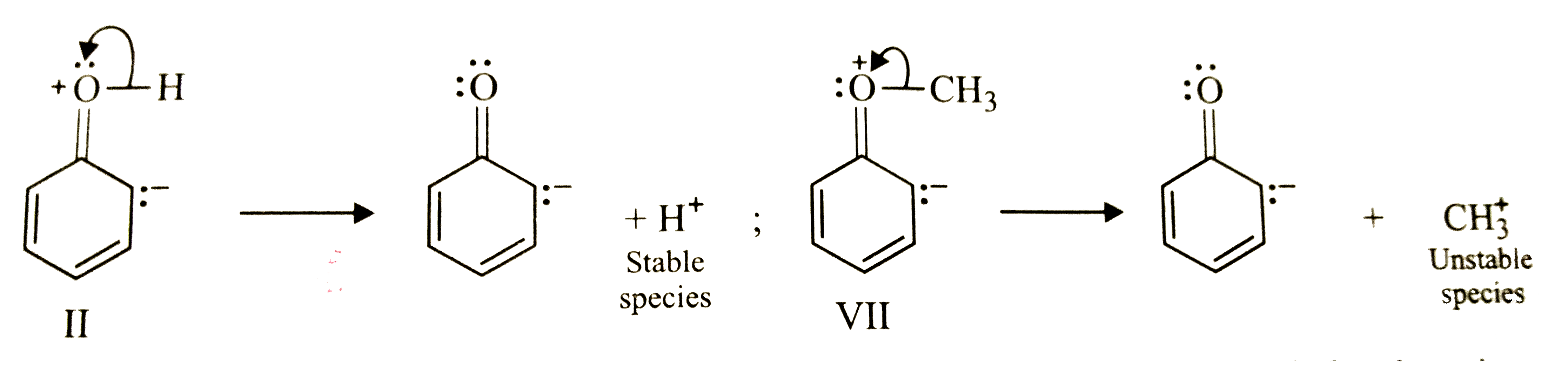
In contrast, structures (VII-IX) cannot stabilize themselves by releasing a methyl carbocation which is a very unstable chemical sepcies. It is because of this extra stability of structures, II-IV through loss of a proton which is responsible for greater reactivity of phenol over anisole. for example, phenol reacts with Br2 water to give 2,4,6-tribromophenol but anisole does not react with Br2 water at all.
|
Similar Questions
Knowledge Check
The most reactive compound towards electrophilic substitution reaction is
AC6H6BC6H6ClCC6H5−CH3DC6H5−OCH3The most reactive of the following towards electrophilic substitution reaction is
 AIBIICIIIDIV
AIBIICIIIDIVThe most reactive compound towards electrophilic substitution reaction is :-
A B
B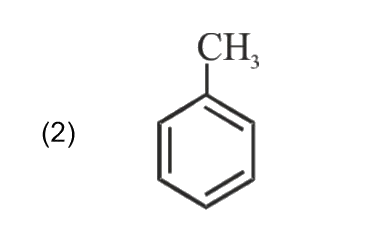 C
C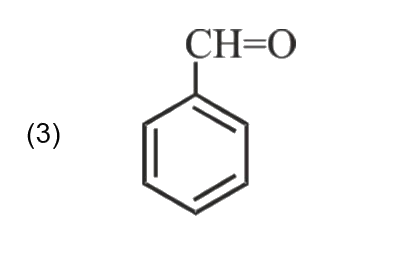 D
D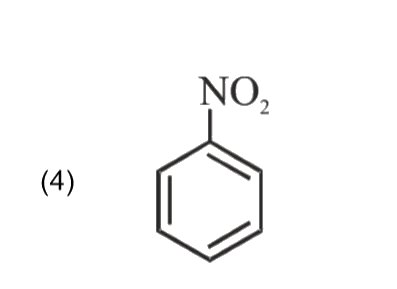
Aryl halide is less reactive than halide towards nucleophilic substitution because
View SolutionTowards electrophilic substitution, the most reactive is
View SolutionThe most reactive compound in electrophilic substitution reaction is
View SolutionWhich of the following is most reactive towards electrophilic substituion reaction ?
View SolutionThe order of reactivity of the following towards electrophillic substitution reaction is
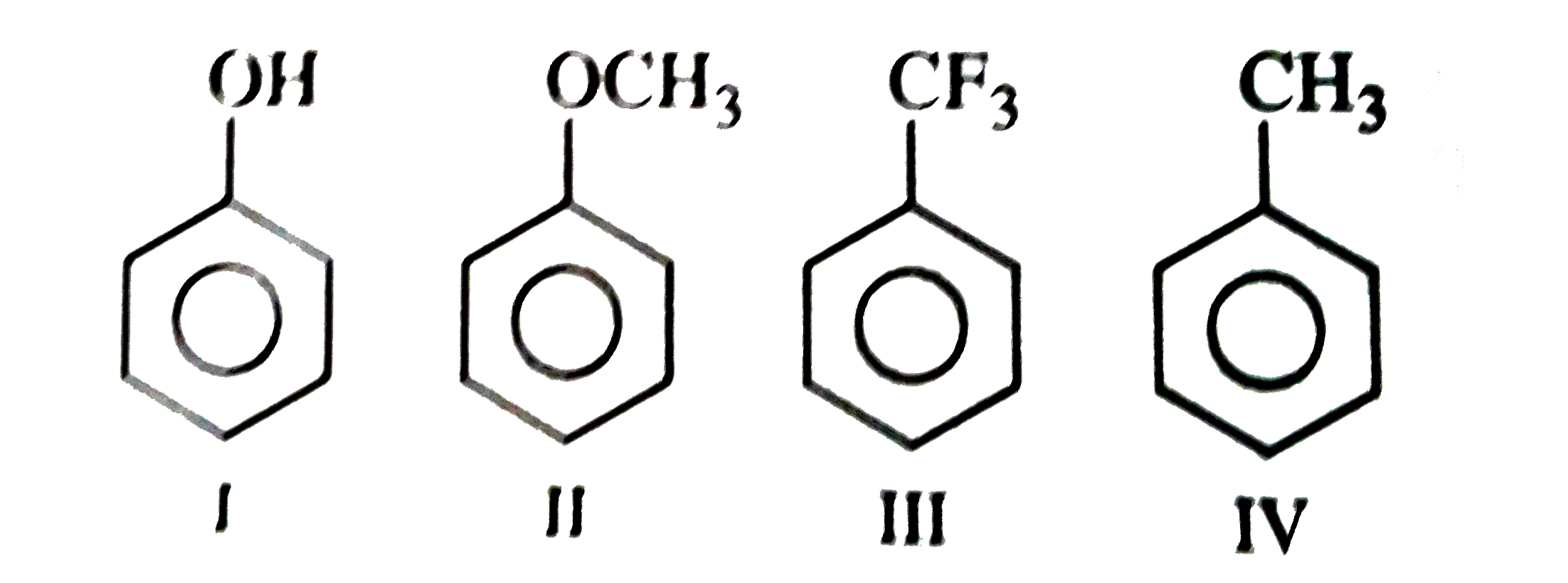
View Solution


