Following compounds are given
(i) CH3CH2OH
(ii) CH3COCH3
(iii) CH3−C∣CH3HOH
(iv) CH3OH
(i), (iiii) and (iv)
only (ii)
(i), (ii) and (iii)
(i) and (ii)
The correct Answer is:C
To determine which of the given compounds can give the Iodoform test, we need to analyze each compound based on its structure and the presence of specific functional groups.
1. Identify the Compounds:
- (i) CH₃CH₂OH - Ethanol
- (ii) CH₃COCH₃ - Acetone
- (iii) CH₃−CH(OH)−CH₃ - 2-Propanol
- (iv) CH₃OH - Methanol
2. Understanding the Iodoform Test:
- The Iodoform test is used to identify the presence of methyl ketones (R-CO-CH₃) or alcohols that can be oxidized to methyl ketones. The positive result is indicated by the formation of a yellow precipitate of iodoform (CHI₃).
3. Analyzing Each Compound:
- Ethanol (CH₃CH₂OH): Ethanol has a primary alcohol structure. When oxidized, it can form acetaldehyde (which is not a methyl ketone), thus it does not give a positive Iodoform test.
- Acetone (CH₃COCH₃): Acetone is a methyl ketone. It will give a positive Iodoform test.
- 2-Propanol (CH₃-CH(OH)-CH₃): 2-Propanol is a secondary alcohol that can be oxidized to acetone (a methyl ketone). Thus, it will also give a positive Iodoform test.
- Methanol (CH₃OH): Methanol is a primary alcohol and does not have the structure to form a methyl ketone upon oxidation. Therefore, it does not give a positive Iodoform test.
4. Conclusion:
- The compounds that can give the Iodoform test are:
- Acetone (ii)
- 2-Propanol (iii)
Final Answer:
The compounds that can give the Iodoform test are (ii) Acetone and (iii) 2-Propanol.
- (i) CH₃CH₂OH - Ethanol
- (ii) CH₃COCH₃ - Acetone
- (iii) CH₃−CH(OH)−CH₃ - 2-Propanol
- (iv) CH₃OH - Methanol
- The Iodoform test is used to identify the presence of methyl ketones (R-CO-CH₃) or alcohols that can be oxidized to methyl ketones. The positive result is indicated by the formation of a yellow precipitate of iodoform (CHI₃).
- Ethanol (CH₃CH₂OH): Ethanol has a primary alcohol structure. When oxidized, it can form acetaldehyde (which is not a methyl ketone), thus it does not give a positive Iodoform test.
- Acetone (CH₃COCH₃): Acetone is a methyl ketone. It will give a positive Iodoform test.
- 2-Propanol (CH₃-CH(OH)-CH₃): 2-Propanol is a secondary alcohol that can be oxidized to acetone (a methyl ketone). Thus, it will also give a positive Iodoform test.
- Methanol (CH₃OH): Methanol is a primary alcohol and does not have the structure to form a methyl ketone upon oxidation. Therefore, it does not give a positive Iodoform test.
- The compounds that can give the Iodoform test are:
- Acetone (ii)
- 2-Propanol (iii)
The compounds that can give the Iodoform test are (ii) Acetone and (iii) 2-Propanol.
Similar Questions
Name the following compounds according to IUPAC system. (i) CH_(3) - CH_(2) - underset (CH_(2)Cl) underset(|)(CH) - overset (CH_(2)OH) overset(|) (CH) - underset (CH_(3)) underset (|) (CH) - CH_(3) CH_(3)- underset (CH_(3)) underset(|) (CH) -CH_(2) - underset(OH) underset(|) (CH) - overset (CH_(2)OH) overset(|) (CH) - CH_(2) (iii) (iv) H_(2)C = CH - underset (OH)underset (|) (CH) - CH_(2) - CH_(2) - CH_(3) (v) CH_(3) - underset (CH_(3)) underset ( |) C = underset (Br)underset(|) C - CH_(2)OH
View SolutionName the following compounds accoring to IUPAC system : (i) CH_(3)-CH_(2)- underset(CH_(2)Cl) underset(|)(CH)-overset(CH_(2)OH) overset(|)(CH)-underset(CH_(3)) underset(|)(CH)-CH_(3) (ii) CH_(3)-underset(CH_(3))underset(|)(CH)-CH_(2)-underset(OH) underset(|)(CH)-overset(CH_(2)OH) overset(|)(CH)-CH_(3) (iii) (iv) H_(2)C=CH-underset(OH)underset(|)(CH)-CH_(2)-CH_(2)-CH_(3) (v) CH_(3)- underset(CH_(3)) underset(|)(C)= underset(Br) underset(|)(C)-CH_(2)OH
View SolutionWrite IUPAC names of the following compounds :
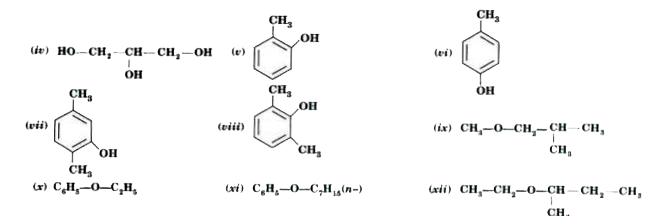
(i) CH3−CH∣CH3−CH2−CH∣OH−CH3∣C∣CH3−CH3 (ii) H3−C−CH∣OH−CH2−CH∣OH−CH∣C2H5−CH2−CH3
CH3−CH∣OH−CH∣OH−CH3
(iv) HO−CH2−CH∣OH−CH2−OH
View SolutionI) CH_(3)-CH_(2)-CH_(2)CH_(2)-OH II) CH_(3)-CH_(2)-underset(OH)underset(|)CH-CH_(3) III) CH_(3)-underset(CH_(3))underset(|)overset(OH)overset(|)C-CH_(3) Among these , III is the chain isomer of
View SolutionI. CH3−CH2−CH2−CH2−OH
II. CH3−CH2−CH∣OH−CH3
III. CH3−CH3∣C∣OH−CH3
IV. CH3−CH∣CH3−CH2−OH
V. CH3−CH2−O−CH2−CH3
VI. CH3−O−CH2−CH2−CH3
VII. CH3−O−CH∣CH3−CH3
Which of the above compounds form pairs of metamers ?
View SolutionI. CH3−CH2−CH2−CH2−OH
II. CH3−CH2−CH∣OH−CH3
III. CH3−CH3∣C∣OH−CH3
IV. CH3−CH∣CH3−CH2−OH
V. CH3−CH2−O−CH2−CH3
VI. CH3−O−CH2−CH2−CH3
VII. CH3−O−CH∣CH3−CH3
Identify the pairs of compounds which are functional group isomers
View SolutionI. CH3−CH2−CH2−CH2−OH
II. CH3−CH2−CH∣OH−CH3
III. CH3−CH3∣C∣OH−CH3
IV. CH3−CH∣CH3−CH2−OH
V. CH3−CH2−O−CH2−CH3
VI. CH3−O−CH2−CH2−CH3
VII. CH3−O−CH∣CH3−CH3
Identify the pairs of compounds that represents position isomerism.
View SolutionI. CH_(3) - CH_(2) - CH_(2) - CH_(2) - OH II. CH_(3) - CH_(2) - underset(OH)underset(|)(CH) - CH_(3) III. CH_(3) - underset(OH) underset(|)overset(CH_(3))overset(|)(C) - CH_(3) IV. CH_(3) - underset(CH_(3))underset(|)(CH) - CH_(2) - OH V. CH_(3) - CH_(2) - O - CH_(2) - CH_(3) VI. CH_(3) - O - CH_(2) - CH_(2) - CH_(3) VII. CH_(3) - O - underset(CH_(3))underset(|)(CH) - CH_(3) Identify the pair of compounds that represents chain isomerism
View Solution

