Assertion : Aromatic aldehydes and formaldehyde undergo Cannizzaro reaction
Reason : Aromatic aldehydes are almost as reactive as formaldehyde.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statement.
Assertion is correct statement but reason is wrong statement.
Assertion is wrong statement but reason is correct statement.
The correct Answer is:C
Correct reason. Formaldehyde and aromatic aldehydes do not contain α-hydrogen.
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:PRADEEPChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS)
Explore 4 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:PRADEEPChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)
Explore 70 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:PRADEEPChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MATCHING TYPE QUESTIONS)
Explore 2 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.
Explore 29 Videos
- APPENDIXBook:PRADEEPChapter:APPENDIXExercise:MODEL TEST PAPER <br> (Section C )
Explore 15 Videos
Similar Questions
An alkene 'A' (molecular formula C5H10) on ozonolysis gives a mixture of two compounds 'B' and 'C'. Compound 'B' gives positive Fehling's test and also forms iodoform on treatement with I2 and NaOH. Compound 'C' does not give Fehling's test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
View SolutionGive the IUPAC names of: (i) Diacetone alcohol (ii) Crotonaldehyde.
View SolutionMatch the acids given in column I with their correct IUPAC names given in column II.
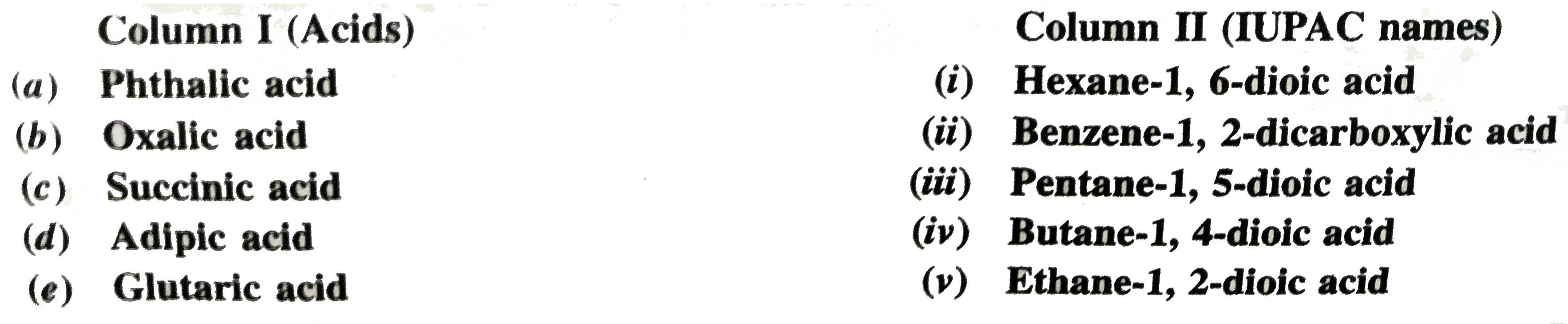 .
.
View SolutionGive the IUPAC name of
View SolutionCalcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
What mass of CaCO3 is required to react completely with 25mL of 0.75MHCl?
View SolutionREACTIVITY OF AROMATIC & ALIPHATIC ALDEHYDE & KETONE
View SolutionAssertion : Aromatic aldehydes and formadehyde undergo Cannizzaro reaction Reason : Aromatic aldehydes are almost reactive a formaldehyde.
View SolutionAssertion:-Aliphatic aldehyde can be diffrentiate from aromatic aldehyde by Fehling solution.
Reason:- This is because aliphatic aldehyde oxidises fehling solution but aromatic aldehyde donot.
View Solution(A) Aromatic aldehydes as well as formaldehyde undergo Cannizzaro's reaction with strong alkali. (R) Aldehydes which have alpha -hydrogen atoms undergo Cannizzaro's reaction.
View SolutionAssertion : Aromatic aldehydes and formaldehyde undergo Cannizzaro reaction.
Reason : Those aldehydes which have α-H atom undergo Cannizzaro reaction.
View Solutionऐरोमैटिक ऐल्डिहाइड व फॉर्मेल्डिहाइड के मध्य क्रॉस (cross) कैनिजारो अभिक्रिया में किसका ऑक्सीकरण होता है?
View SolutionAromatic aldehyde undergoes condensation on heating with an ethanolic solution of KCN. This reaction is called as
View SolutionA : Aromatic aldehydes are less reactive than aliphatic aldehyde towards nucleophilic substitution reaction. R : In case of aromatic aldehyde,+R effect of phenyl ring is present.
View SolutionFormaldehyde [HCHO] Undergoes Cannizzaro reaction: Give reason.
View SolutionFormaldehyde (HCHO] undergoes Cannizzaro reaction : Give reason.
View Solution
