Predict the product of the following reaction:
C6H5−CO−CH3NaOH/I2−−−−−→?+?
To predict the product of the reaction of acetophenone (C₆H₅-CO-CH₃) with sodium hydroxide (NaOH) and iodine (I₂), we can follow these steps:
1. Identify the Reactants: The reactants are acetophenone (C₆H₅-CO-CH₃), sodium hydroxide (NaOH), and iodine (I₂).
2. Recognize the Reaction Type: This reaction is known as the iodoform reaction, which occurs with methyl ketones (like acetophenone) in the presence of a strong base and iodine.
3. Deprotonation: The base (NaOH) will deprotonate the methyl group adjacent to the carbonyl (C=O) in acetophenone. This generates an enolate ion:
C₆H₅−C(=O)CH₃NaOH−−−−→C₆H₅−C(=O)C(−)H₂
4. Iodination: The enolate ion will then react with iodine (I₂). The enolate ion attacks iodine, resulting in the formation of iodoacetophenone:
C₆H₅−C(=O)C(−)H₂+I₂→C₆H₅−C(=O)C(I)H
5. Further Iodination: The iodoacetophenone can undergo further iodination. The remaining hydrogen on the methyl group can be replaced by iodine, leading to the formation of tri-iodoacetophenone:
C₆H₅−C(=O)C(I)H+I₂→C₆H₅−C(=O)C(I)I
6. Formation of Iodoform: The final step involves the cleavage of the carbon chain to produce iodoform (CHI₃) and sodium benzoate (C₆H₅COONa):
C₆H₅−C(=O)C(I)I→CHI₃+C₆H₅COONa
Final Products:
The products of the reaction are:
- Iodoform (CHI₃), which is a yellow solid.
- Sodium benzoate (C₆H₅COONa), which is the salt of benzoic acid.
Summary of the Reaction:
C₆H₅−CO−CH₃+NaOH+I₂→CHI₃+C₆H₅COONa
C₆H₅−C(=O)CH₃NaOH−−−−→C₆H₅−C(=O)C(−)H₂
C₆H₅−C(=O)C(−)H₂+I₂→C₆H₅−C(=O)C(I)H
C₆H₅−C(=O)C(I)H+I₂→C₆H₅−C(=O)C(I)I
C₆H₅−C(=O)C(I)I→CHI₃+C₆H₅COONa
The products of the reaction are:
- Iodoform (CHI₃), which is a yellow solid.
- Sodium benzoate (C₆H₅COONa), which is the salt of benzoic acid.
C₆H₅−CO−CH₃+NaOH+I₂→CHI₃+C₆H₅COONa
Similar Questions
Knowledge Check
Predict the product of the following reaction
C6H5MgBr+CH3NH2→ .AC6H6BCH4CC6H5NHCH3DA mixture of all thesePredict the product of the following reaction C6H5CH2I1.CH3NH2,heat−−−−−−−−−−→2.H2,Ni .
AC6H5NH2B(C6H5)NHC(CH3)2NHDC6H5NHCH3Predict the end product of the following sequence of reactions
C6H5NH2CH3CI−−−→ACH3CI−−−→B .A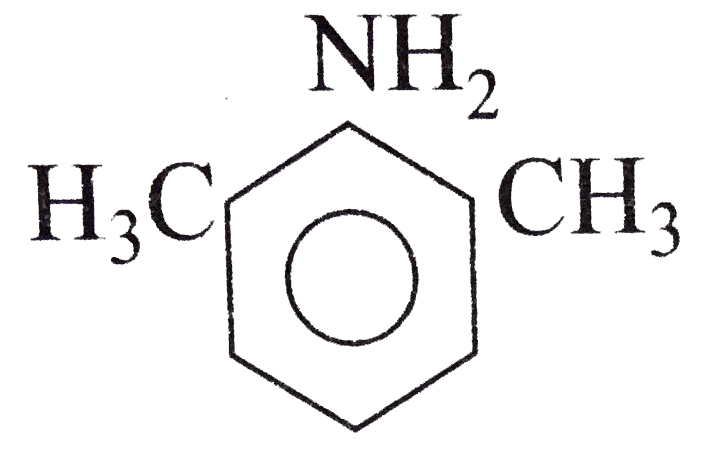 B
B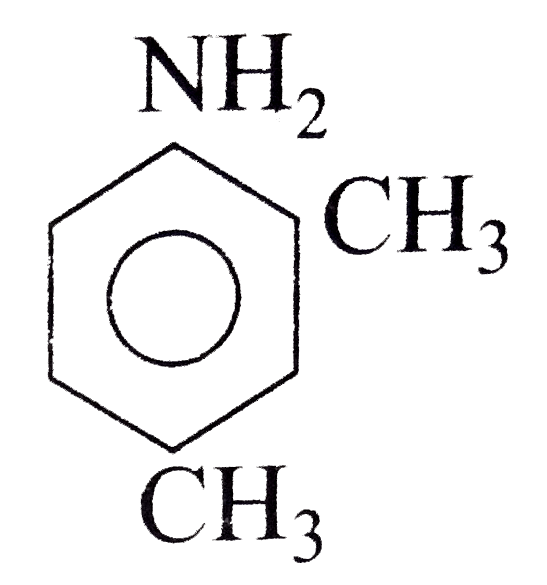 C
C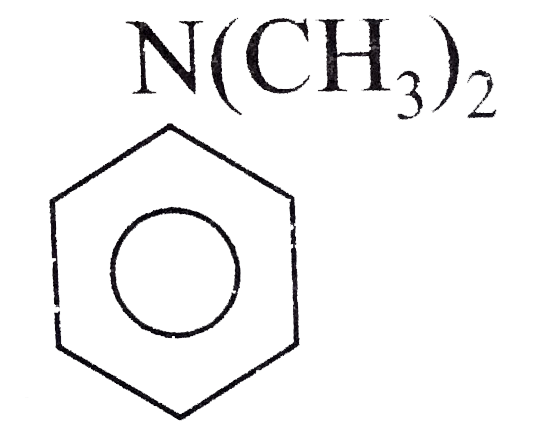 DA mixture of all there
DA mixture of all there
Predict the product of the following reaction CH3NO2+C6H5CHOKOH(alc.)−−−−−−−→
View SolutionPredict the product of the following reaction
CH3COONaNaOH/CaO−−−−−−−→Δ?
View SolutionPredict the major product in the following reaction :
C6H5−CH2CO2CH3(i)CH3MgBr(Excess)−−−−−−−−−−−−−−→(ii)H⊕.
View SolutionPredict the products of the following reactions :
(i) CH3−CH=CH2(i)B2H6−−−−−−−−−−−−→(ii)3H2O2/OH−
(ii) C6H5−OHBr2(aq)−−−−→
(iii) CH3−CH2−OHCu/573K−−−−−→
View SolutionPredict the major product of the following reaction: C6H6+(CH3)2CHCH2OHH2SO4−−−→
View Solution
