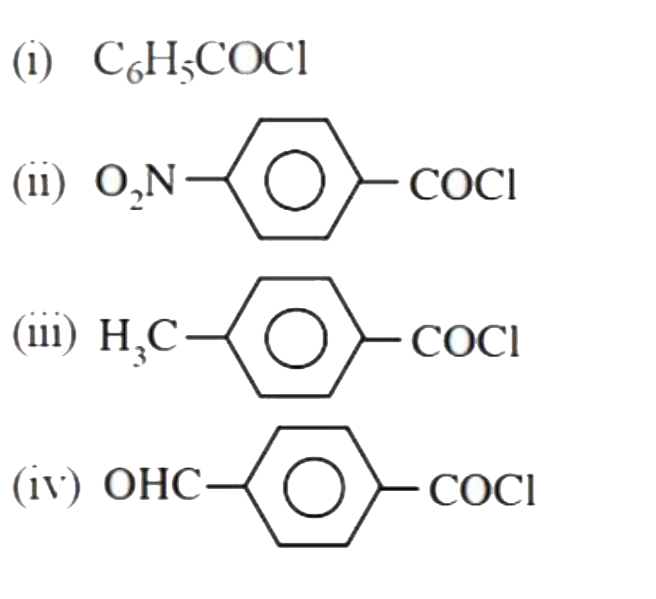
Consider the following compounds:
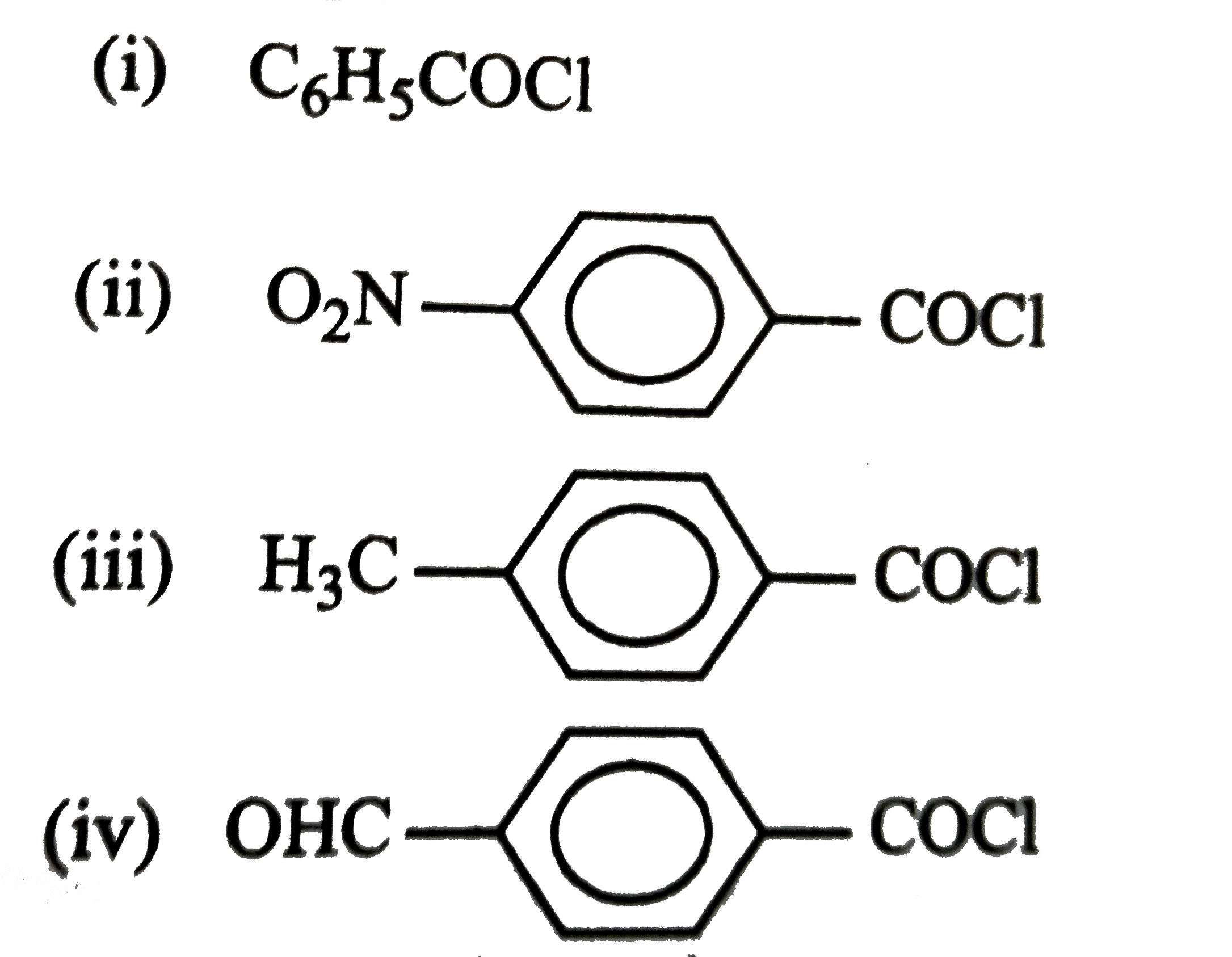
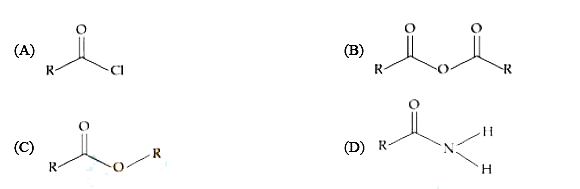
The correct decreasing order of their reactivity towards hydrolysis is:
Video Solution
Text Solution
The correct Answer is:A
Electron-donating groups decrease while electron withdrawing groups increase the reactivity of acid chlorides towards hydrolysis. Thus, option (a) is correct.
|
Similar Questions
Knowledge Check
Consider the following compounds :
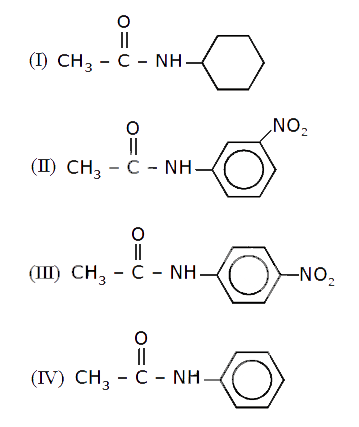
The decreasing order of reactivity towards hydrolysis by aqueous NaOH is :AI gt II gt III gt IVBIII gt II gt IV gt ICIV gt I gt II gt IIIDI gt IV gt II gt IIIConsider the following compunds
(i) C6H5COCl
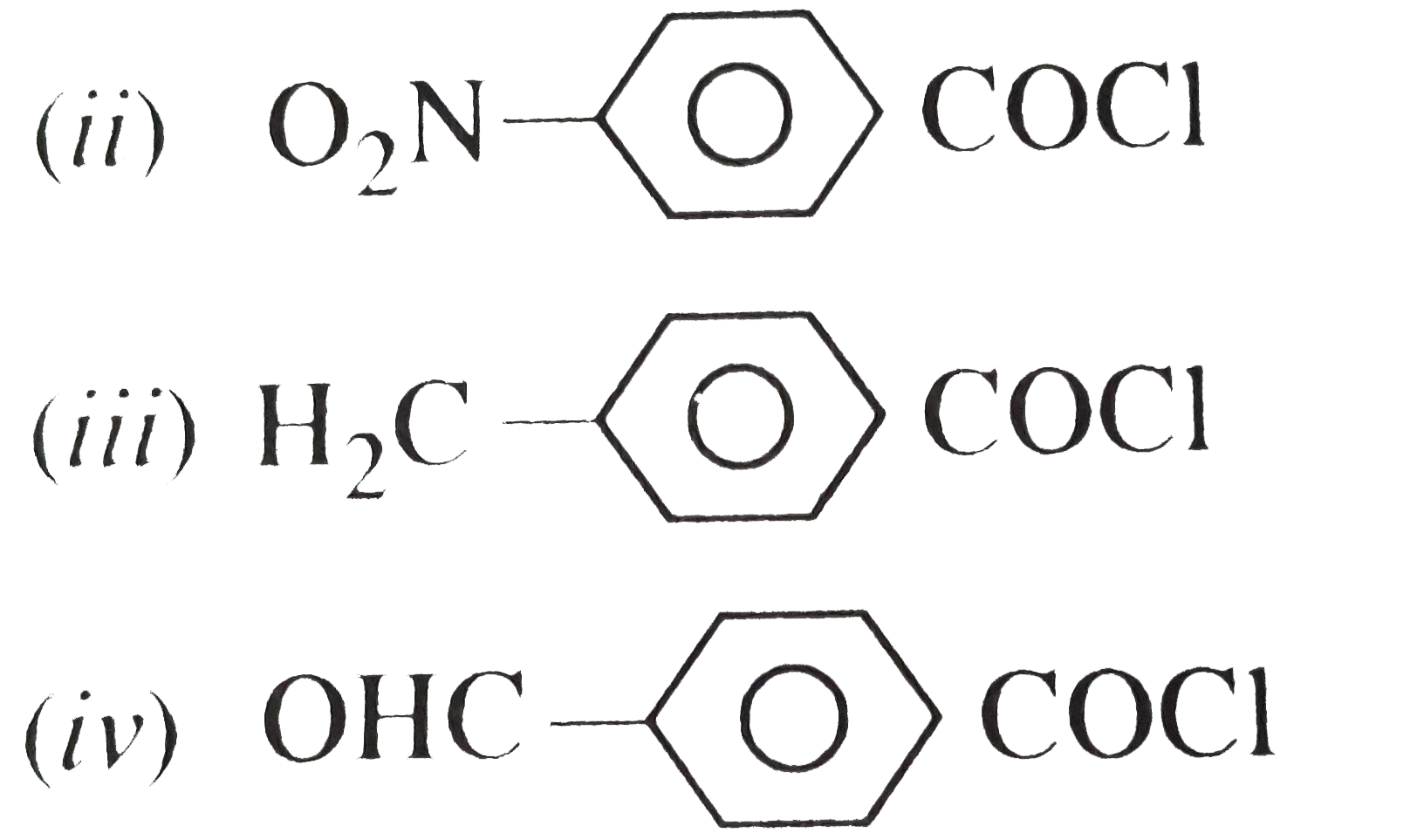
the correct decreasing order of their reactivity towards hydrolysis isAii>iv>i>iiiBii>iv>iii>iCi>ii>iii>ivDiv>ii>i>iiiArrange the following compounds in the decreasing order of their reactivity towards SN2 reaction :
 Aiv>iii>i>iiBii>i>iii>ivCi>ii>iv>iiiDiii>ii>i>iv
Aiv>iii>i>iiBii>i>iii>ivCi>ii>iv>iiiDiii>ii>i>iv
Among the following compounds, the decreasing order of reactivity towards electrophilic substitution is :
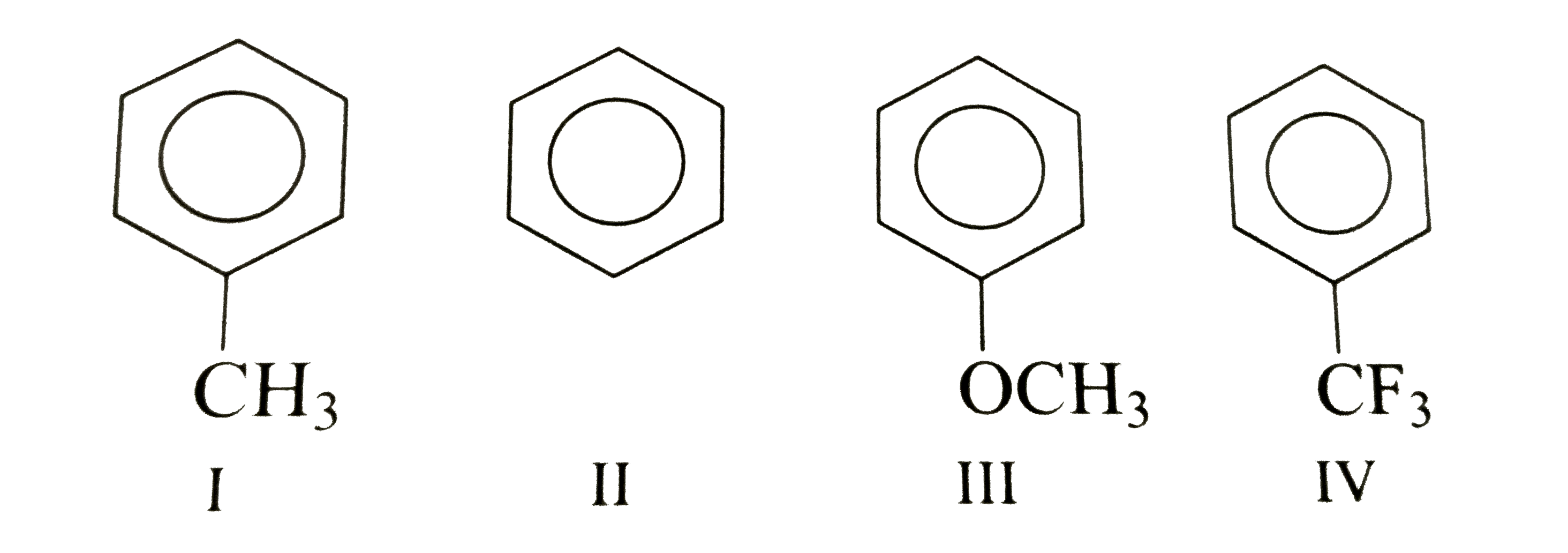
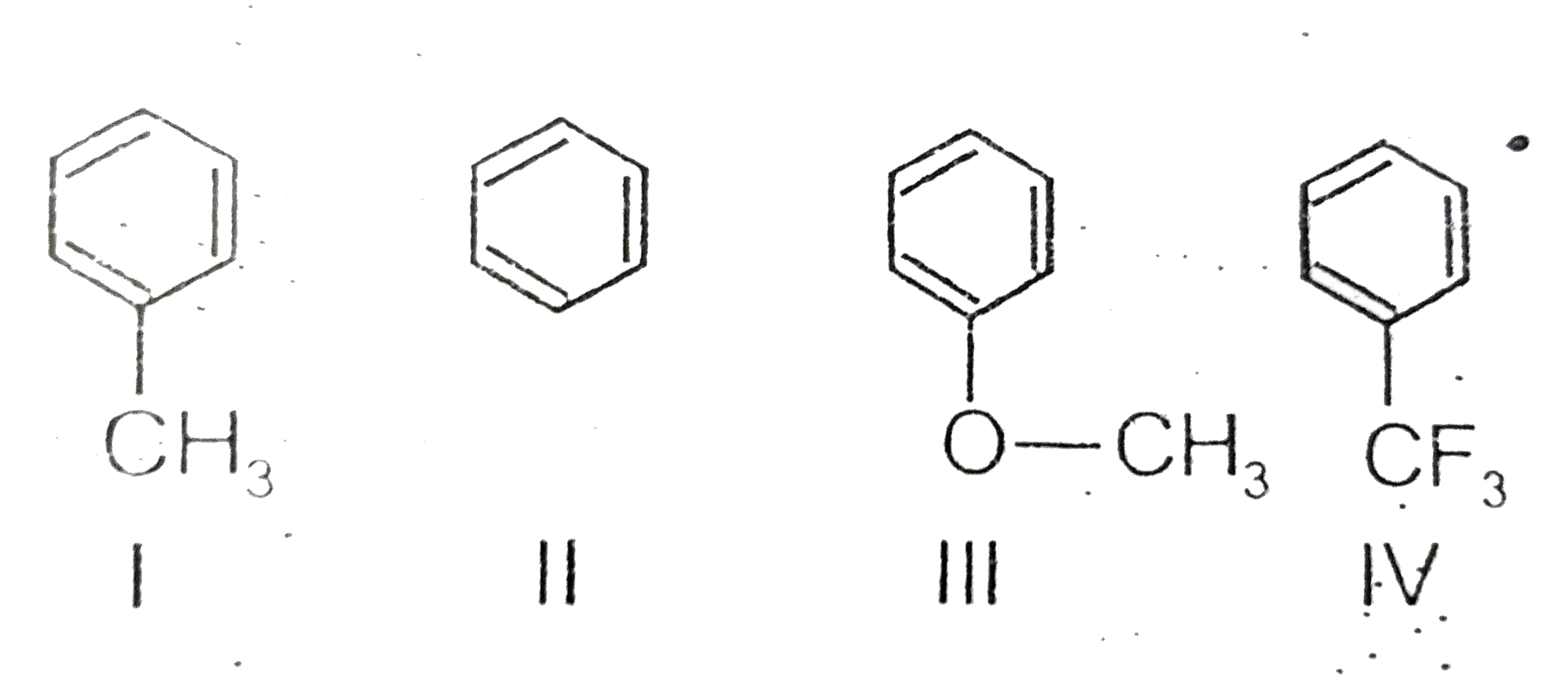
View SolutionAmong the following compounds, the decreasing order of reactivity towards electrophilic substitution is
View SolutionArrange the following compounds in the decreasing order of reactivity for hydrolysis reaction :
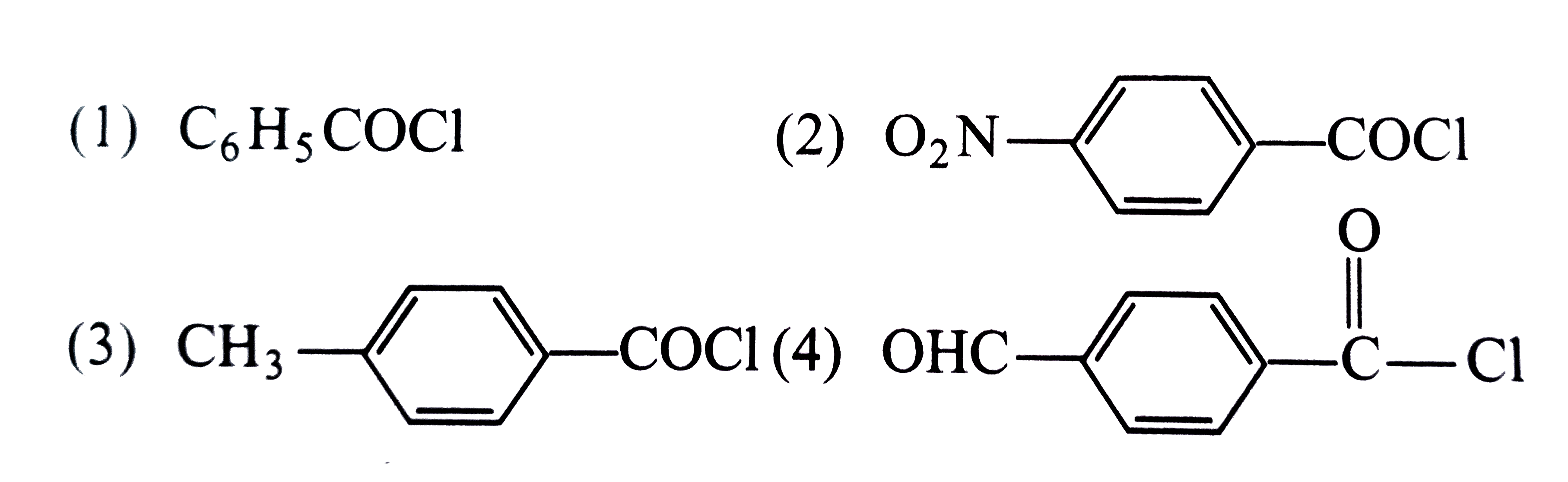
View SolutionConsider the following carbanions :
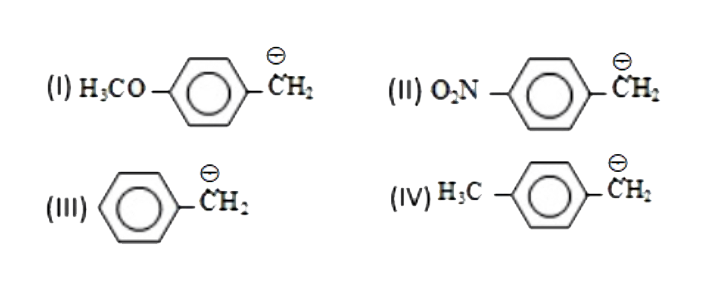
Correct decreasing order of stability is -
View SolutionAmong the following compounds the decreasing order of reactivity towards electrophilic substitution is
View Solution