The presence of a base is needed in the ammonolysis of alkyl halides. Explain.
Video Solution
Text Solution
During ammonolysis of alkyl halides, the acid liberated during the reaction combines with the amine formed to form the amine salt. To liberate free amine from the amine salt, a base is needed. The base used is excess of alcoholic NH3 which serves both as a nucleophile as well as a base. for example.
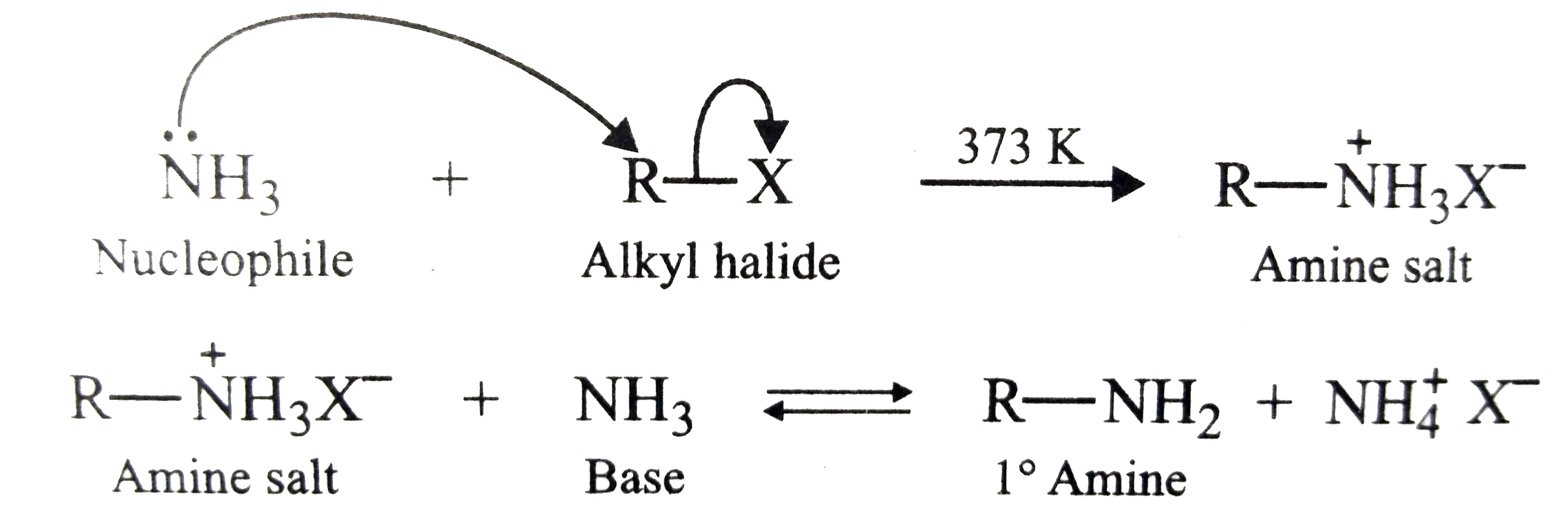 .
.
|
Similar Questions
(a) Give plausible explanation for each of the following :
(i) The presence of a base is needed in the ammonolysis of alkyl halides.
(ii) Aromatic primary amines cannot be prepared by Gabriel phthalimide synthesis.
(b) Write the IUPAC name of
CH3−N−C∣C2H5∣∣O−CH3
View SolutionWhat is obtained finally by continued ammonolysis of alkyl halide ?
View Solution
Knowledge Check
Alkyl halides are :
AMonohalogen derivatives of alkanesBDihalogen derivatives of alkanesCTrihalogen derivatives of alkanesDTetrahlogen derivatives of alkanesAlkyl halide are-
AMono halogen derivatives of alkanesBTrihalogen derivatives of alkanesCDi halogen derivatives of alkanesDTetra halogen derivatives of alkanesWhat is false for alkyl halides?
AThese are completely soluble in waterBThese give nucleophilic substitution reactionsCThese are insoluble in waterDThese are soluble in organic solvents

