Among the following amines, the strongest Bronsted base is….
Video Solution
Text Solution
The correct Answer is:B
In (c), electrons on the N atom are delocalized over two double bonds but in (d), electrons are more strongly delocalized over the benzene ring but in (b), electrons are not delocalized at all, therefore, it is the strongest base. Further, being a 2∘ amine, it is more basic than even NH3. thus, option (b) is correct.
|
Similar Questions
Knowledge Check
Which of the following is the strongest Bronsted base?
A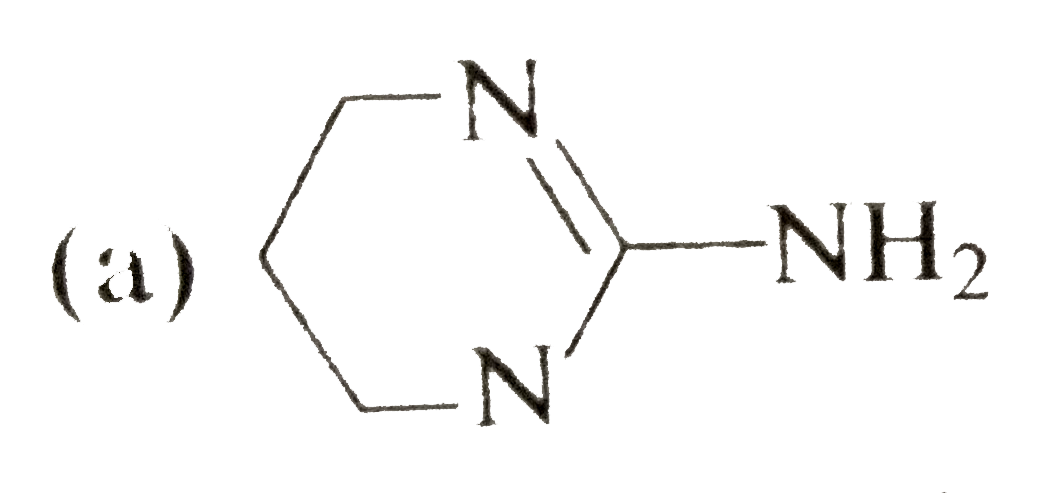 B
B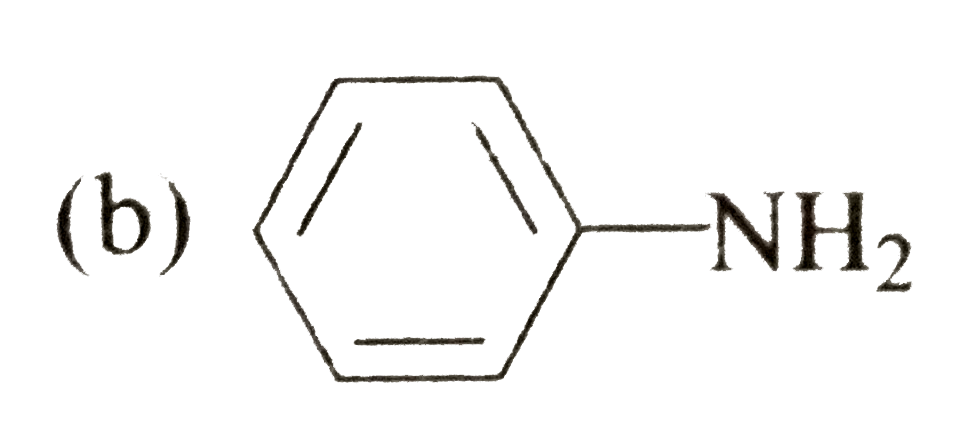 C
C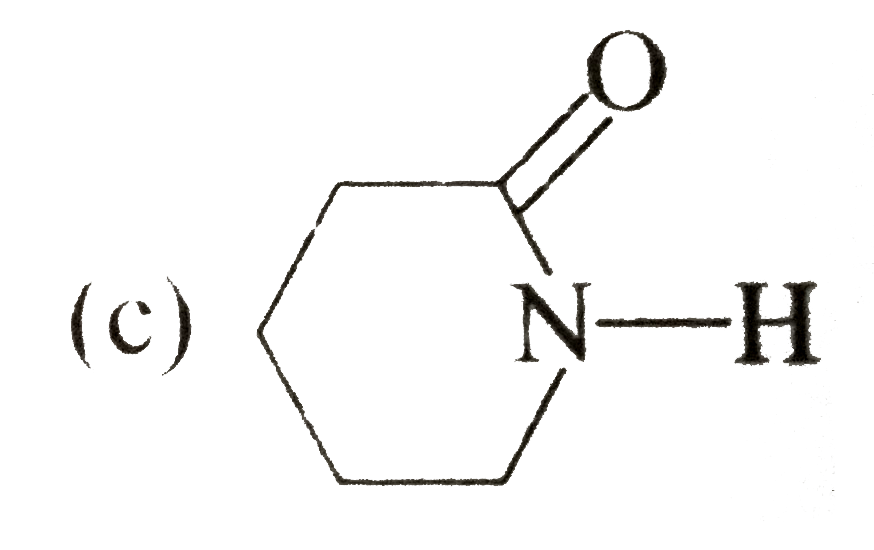 D
D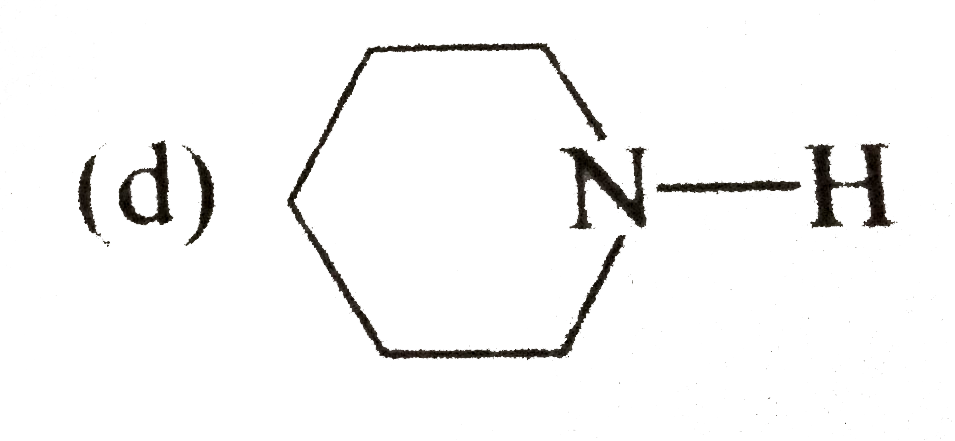
Which of the following is the strongest Bronsted base ?
A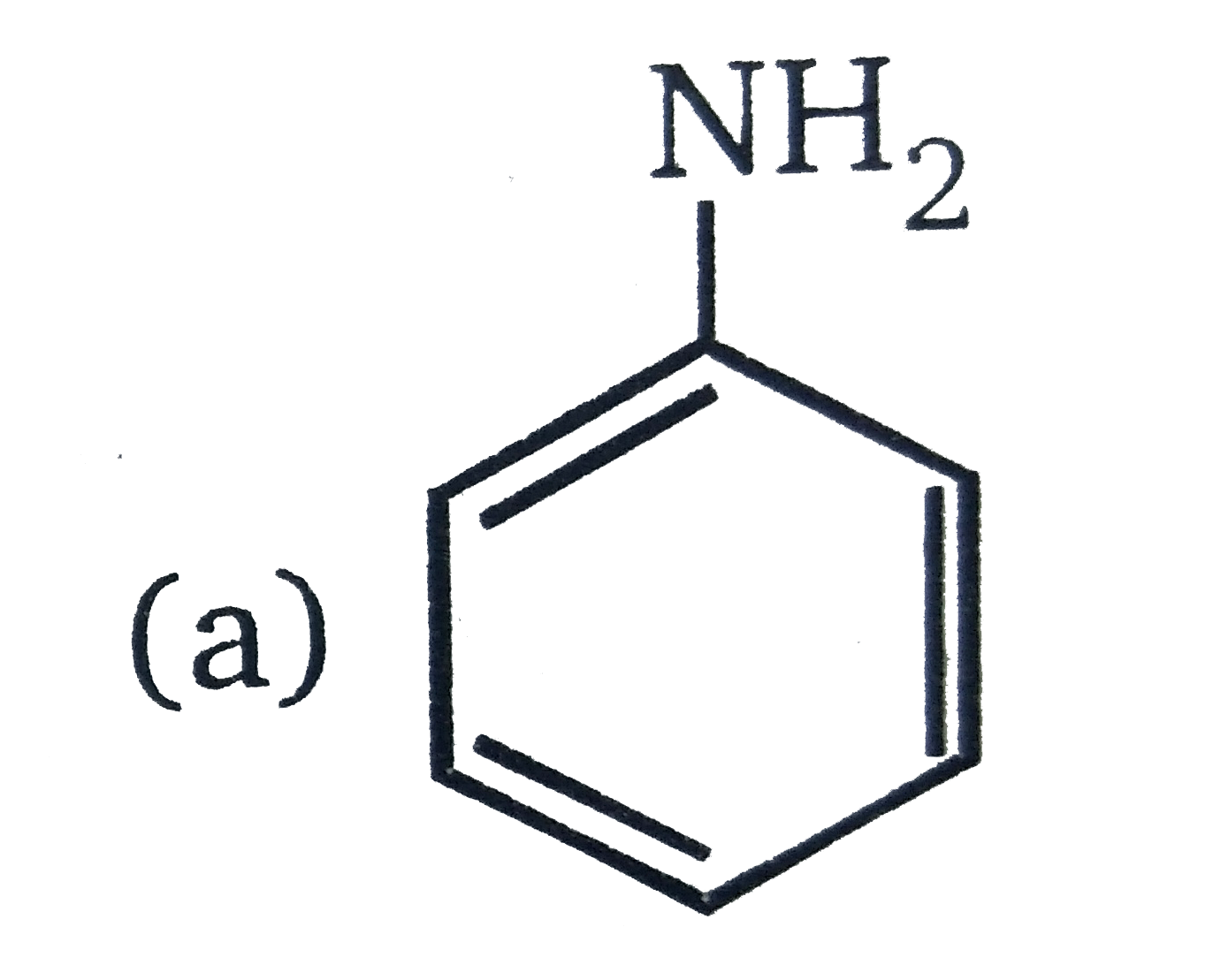 B
B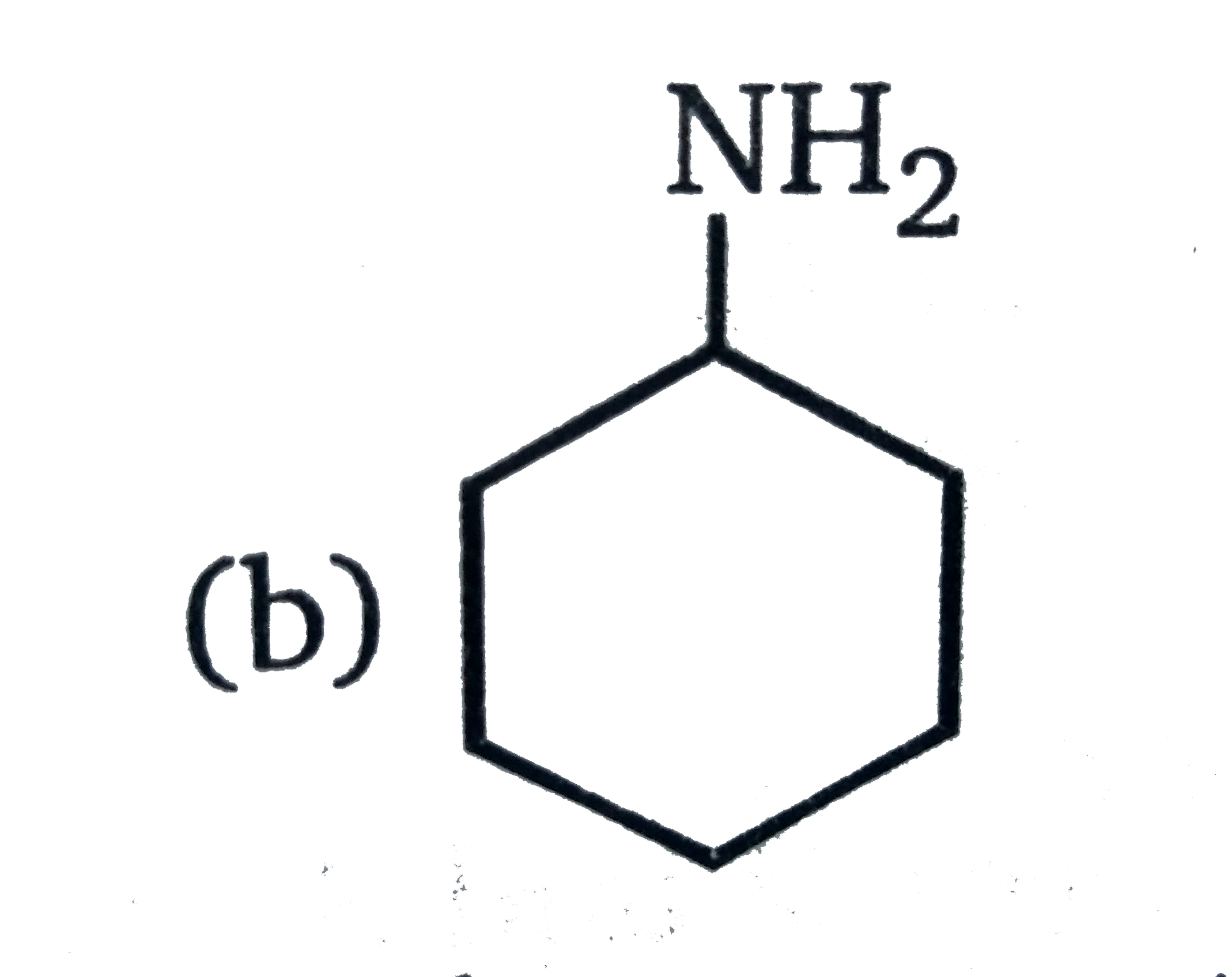 C
C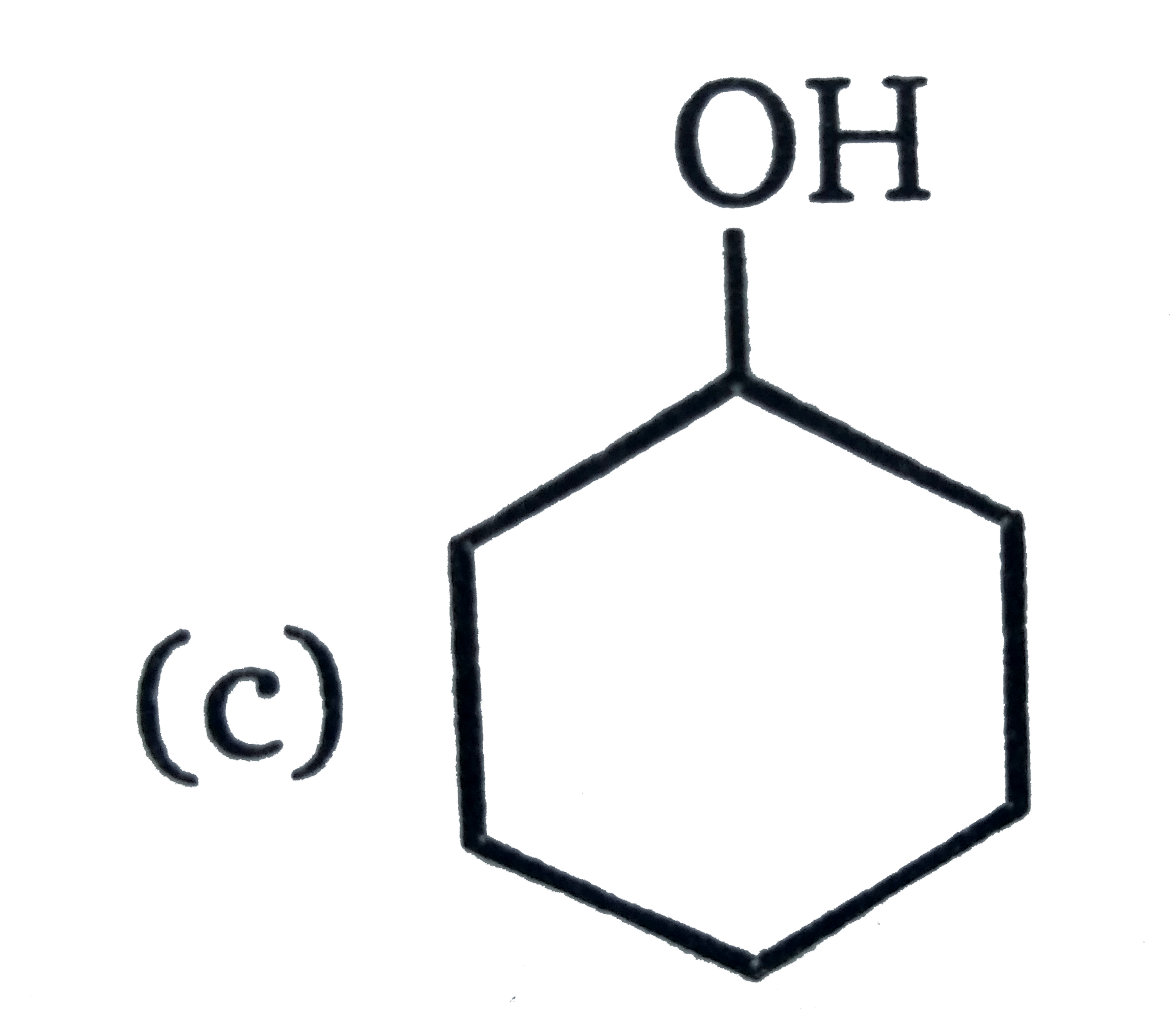 D
D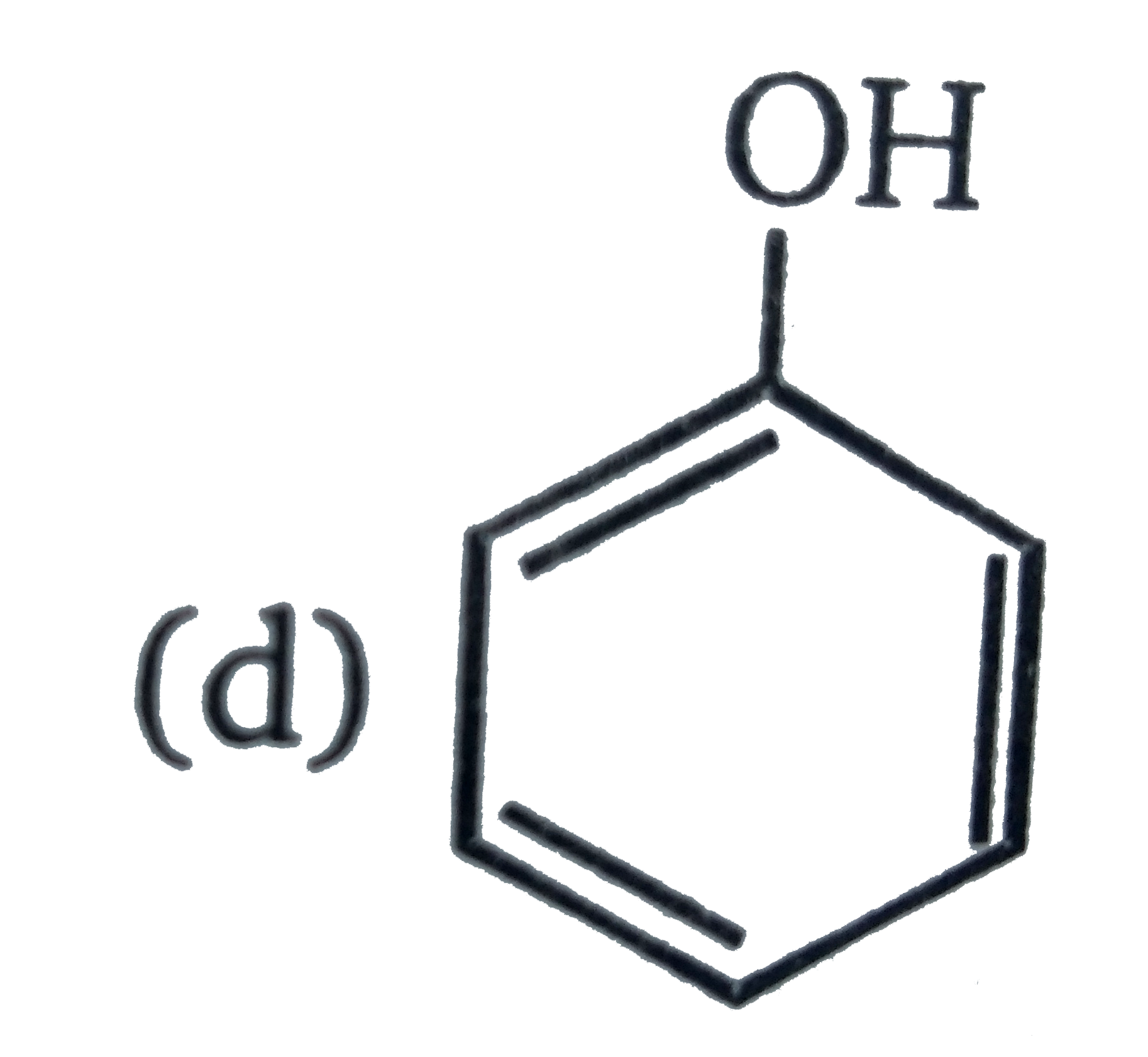
Which one of the following anionc is the strongest Bronsted base ?
ACIO−BCIO−2CCIO−3DCIO−4
Among the following the strongest base is
View SolutionWhich of the following is strongest Bronsted base
View SolutionOf the given anions, the strongest Bronsted base is
View SolutionQuestion Stimulus :-
Of the following the strongest Bronsted base is-
View SolutionOf the given anions, the strongest Bronsted base is
View Solution
