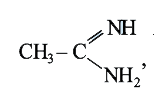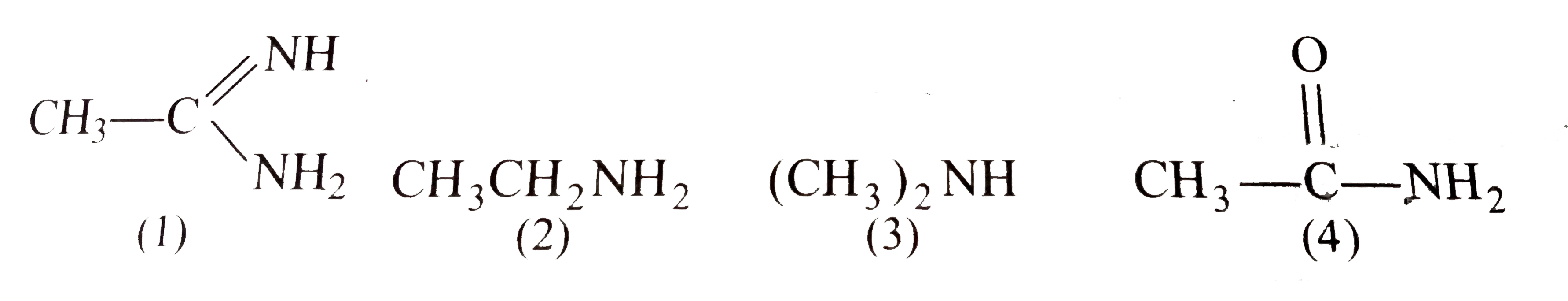The correct order of basicities of the following compounds is
Video Solution
Text Solution
The correct Answer is:B
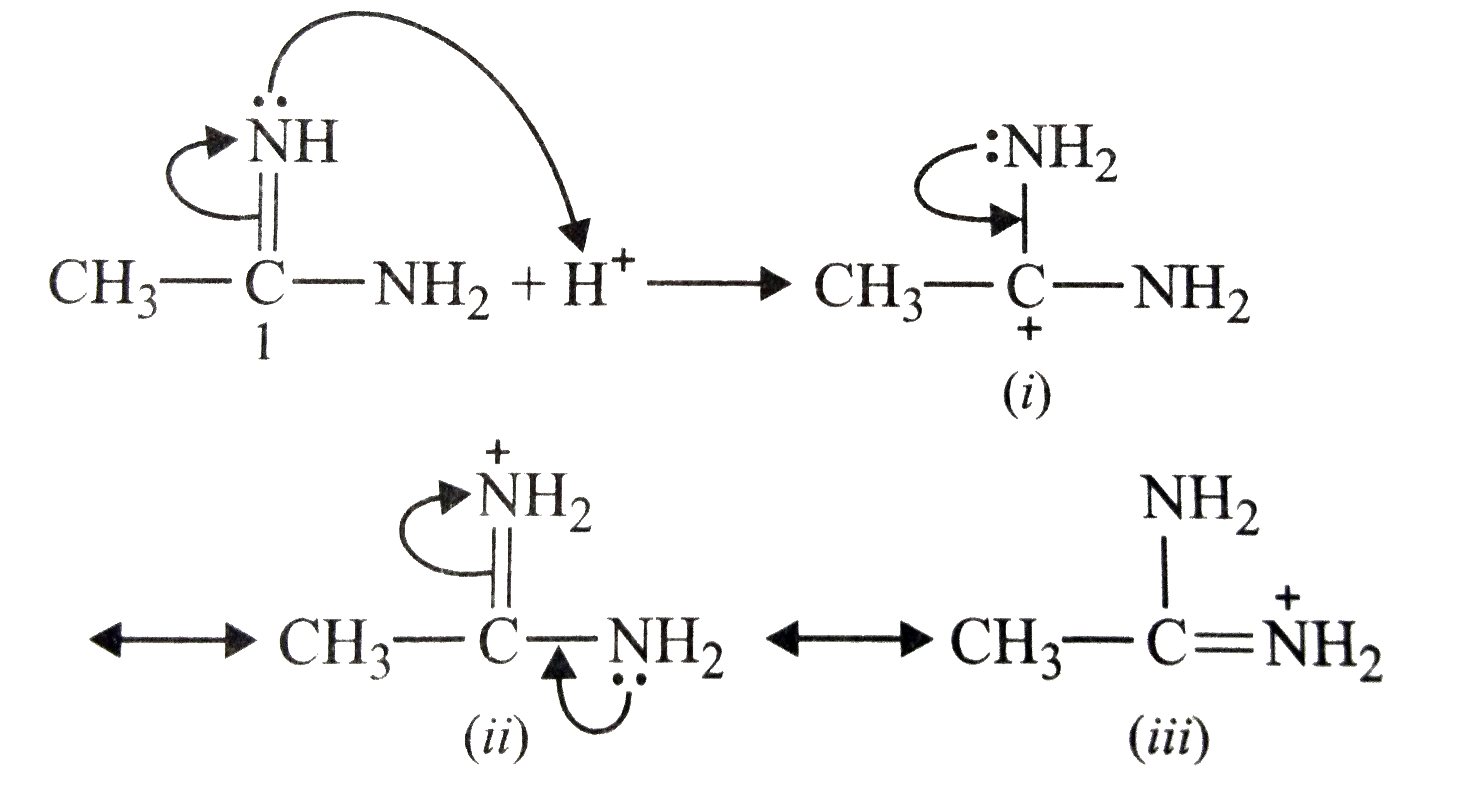
The conjugate acid (i) obtained by addition of a proton to 1 is stabilized by two equivalent resonance structures and hence compound (1) is the most basic. Further, 2∘ amines are more basic than 1∘ amines while amides are least basic due to delocalization of lone pair of electrons of N over the C=O group. thus, the order: 1gt3gt2gt4, i.e., option (b) is correct.
|
Similar Questions
Knowledge Check
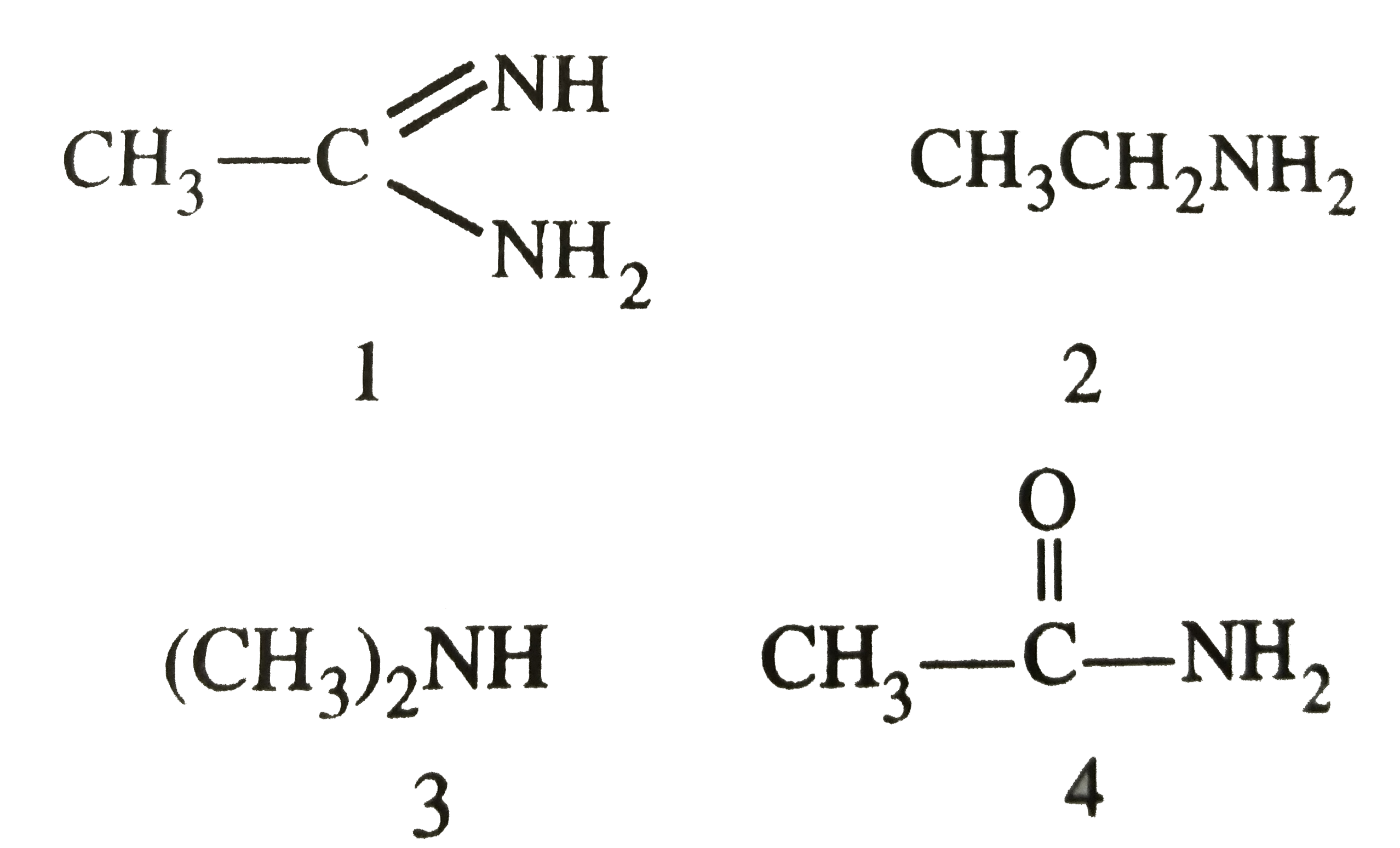
The correct orderr of basicities of the following compounds is
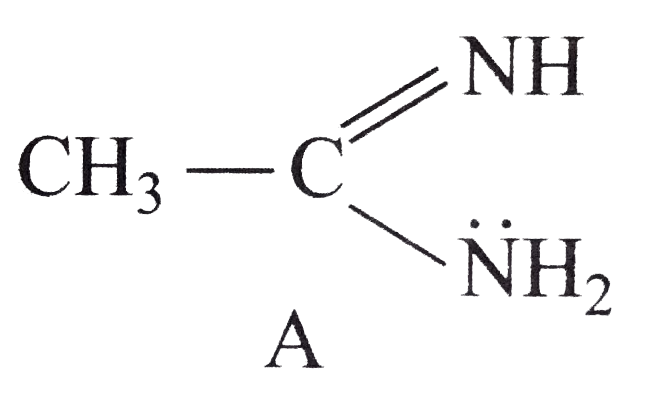
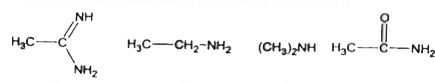 A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4
A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4The correct order of basicity of the following compounds is
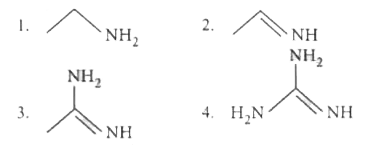
 A1<2<3<4B1<2<4<3C2<1<3<4D4<3<2<1
A1<2<3<4B1<2<4<3C2<1<3<4D4<3<2<1The correct order of the basicities of the following comounds is .
 .AA>B>C>DBC>A>B>DCB>A>C>DDA>C>B>D
.AA>B>C>DBC>A>B>DCB>A>C>DDA>C>B>D
The correct order of basicity of the following compouds is
View SolutionThe order of basicity of the following compounds is
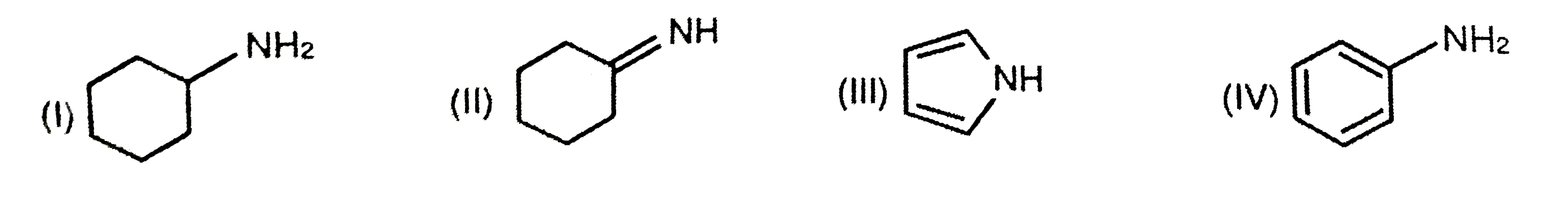
View SolutionThe order of basicity of the following compounds is
View SolutionThe correct order of basicity of the following compound in aqueous medium is
(1) NH3 (2) CH2CH2NH2
(3) (CH3)2NH (4) (C2H5)3N
View SolutionThe compound order of basicities of the following compounds is
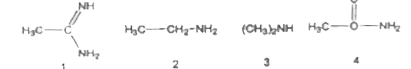
View Solution