Treatment of compound O with KMnO4/H+

gave P, which on heating with ammonia gave Q. the compound Q on treatment with Br2/NaOH produced R. On strong heating, Q gae S, which on further treatment with ehtyl 2-bromopropanoate in the presence of KOH followed by acidification, gave a compound T.
Q. The compoudn R is




The correct Answer is:A
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:PRADEEPChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (IV. MATHING TYPE QUESTIONS)
Explore 1 Video
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:PRADEEPChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (VI. INTEGER TYPE QUESTIONS)
Explore 5 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:PRADEEPChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCE SPECIAL (MULTIPLE CHOICE QUESTIONS-II)
Explore 5 Videos
- HALOALKANES AND HALOARENESBook:PRADEEPChapter:HALOALKANES AND HALOARENESExercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 22 Videos
- P-BLOCK ELEMENTS Book:PRADEEPChapter:P-BLOCK ELEMENTS Exercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 25 Videos
Similar Questions
Match the four starting materials (P,Q,R,S) given in List I with the corresponding reaction schemes (I,II,III,IV) provided in List II and select the correct answer using the code given below the lists.


View SolutionHow many isomeric alkanes having the molecular formula C5H12 are possible?
View SolutionIsopropylamine can be obtained by
View SolutionWrite the structure of 1-Bromo-4-chlorobut-2-ene
View SolutionNO (nitric oxide) is paramagnetic in the gaseous state but diamagnetic in the liquid and solid states. Why ?
View SolutionTwo aliphatic aldehydes (P) and (Q) react in the presence of aqueous K_(2)CO_(3) to give compound (R ) which upon treatment with HCN gives compound(S). On acidification and heating, (S) gives the product shown below: Compounds (P) and (Q), respectively, are:
View SolutionTwo aliphatic aldehydes (P) and (Q) react in the presence of aqueous K2CO3 to give compound (R ) which upon treatment with HCN gives compound(S). On acidification and heating, (S) gives the product shown below:

Compound (R ) is:
View SolutionTreatment of compound o with KMnO4/H+ gave P, which on heating with ammonia gave Q. The compound Q on treatment with Br2/NaOH produced R.On strong heating ,Q gave S, which on furthur treatment with ethyl2-bromopropanate in the presence of KOH followed by acidfication , gave acidificatin , gave a compound T.4

The compound R is
View SolutionTreatment of compound o with KMnO_(4)//H^(+) gave P, which on heating with ammonia gave Q. The compound Q on treatment with Br_(2)//NaOH produced R.On strong heating ,Q gave S, which on furthur treatment with ethyl2-bromopropanate in the presence of KOH followed by acidfication , gave acidificatin , gave a compound T.4 The compound T is
View SolutionTwo aliphatic aldehydes P and Q react in th presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compound S. On acidification and heatingm,S gives the product shown below:
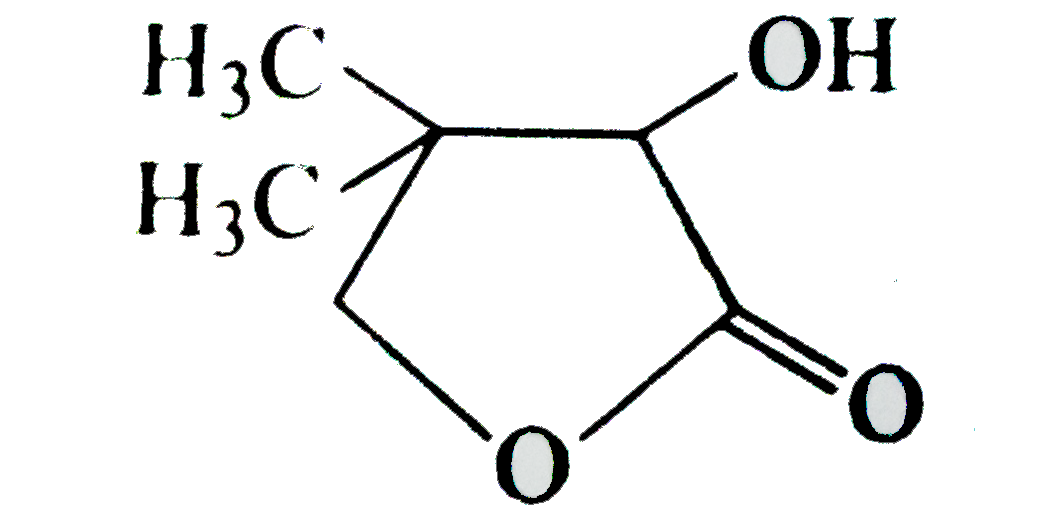
The compounds P and Q respectively are
View SolutionThe reaction of compounf P with CH3MgBr (excess) in (C2H5)2O followed by addition of H2O give Q the compound Q on treatment with H2SO4at 0∘ C gives R. the reaction of R with CH3COCl in the presence of anhydrous AlCl3 in CH2Cl2 followed by treatment with H2O produces compound S. [Et in compound P is ethyl group] The product S is
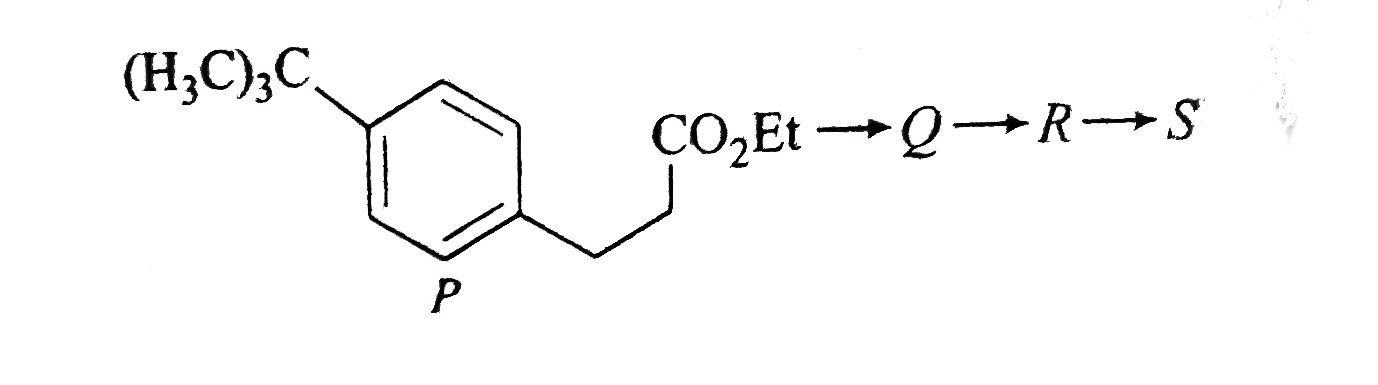
View SolutionTreatment of compound o with KMnO4/H+ gave P, which on heating with ammonia gave Q. The compound Q on treatment with Br2/NaOH produced R.On strong heating ,Q gave S, which on furthur treatment with ethyl2-bromopropanate in the presence of KOH followed by acidfication , gave acidificatin , gave a compound T.4

The compound T is
View SolutionTreatment of compound O with KMnO4/H+ have P, which on heating with ammonia gave Q. The compound Q on treatment with Br2/NaOH produced R. On strong heating, Q gave S, which on further treatment with ethyl 2-bromopropanoate in the presence of KOH followed by acidification, gave a compound T.
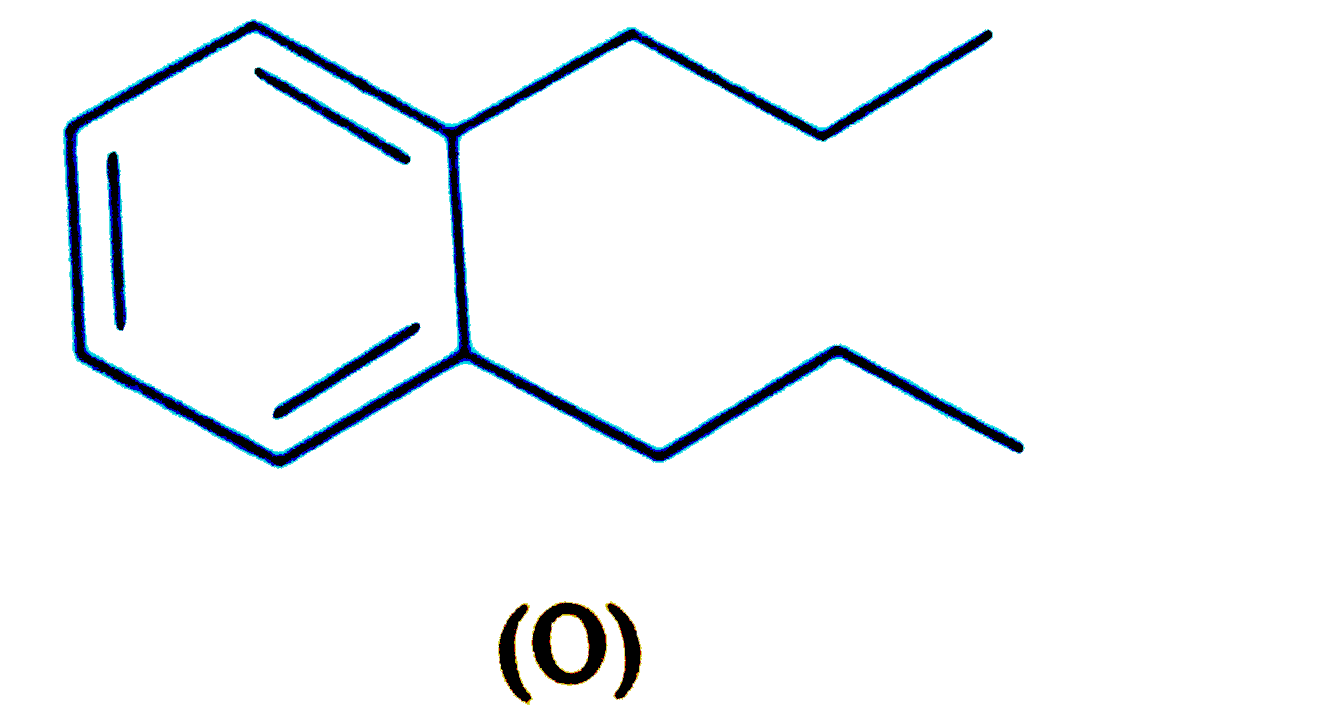
Q. The compound R is
View SolutionTreatment of compound O with KMnO_(4)//H^(+) have P, which on heating with ammonia gave Q. The compound Q on treatment with Br_(2)//NaOH produced R. On strong heating, Q gave S, which on further treatment with ethyl 2-bromopropanoate in the presence of KOH followed by acidification, gave a compound T. Q. The compound T is
View SolutionAn organic compound A containing C = 70% and H =11.6% gave the following results :
(P) 0.384 gm of the compound A displaced 100 ml of air at 1 atm and 273 K.
(Q) On treatment with PCl3A gave another compound, which contained 33.97%(34%)chlorine.
IUPAC anme of the compound A is :
View SolutionPropene was oxidised by aqueous KMnO_(4) to give a compound (A), Treatment of compound (A) with thionyl chloride gave:
View Solution


