Free radical polymerization of stryene gives a product in which groups are on alternate carbon atoms rather than on adjacent carbon atoms. Exlpain.
Text Solution
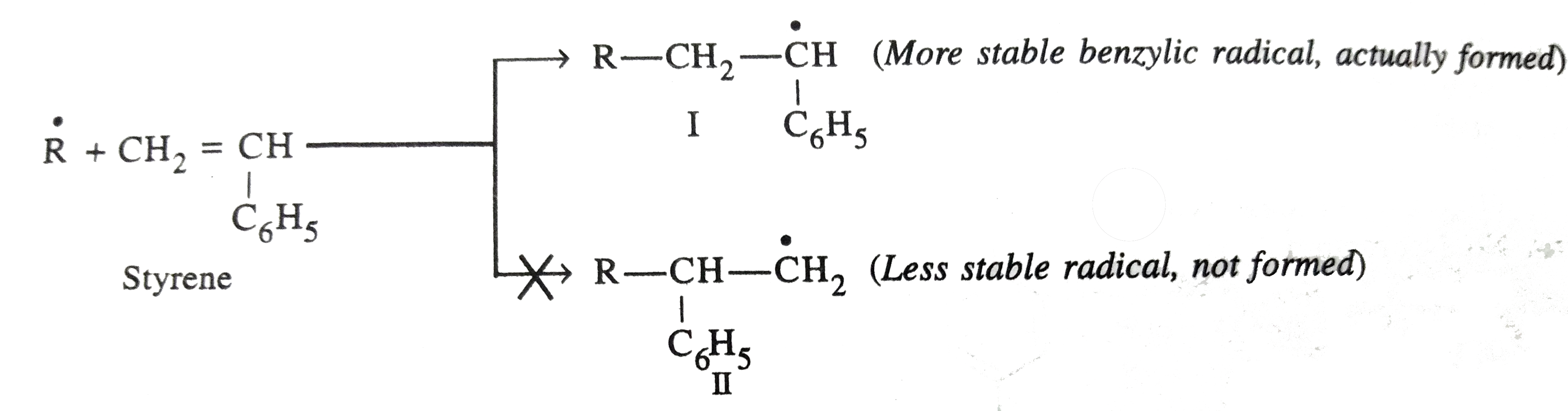
During free radical polymerization , the addition of free radicals to monomer molecules occurs in accrodance with Markovnikov's rule so as to give more stable free radical. For example , addition of R. (obtained from radical initiator) to styrene gives more stable benzylic radical (I) rather than less stable radical (II).
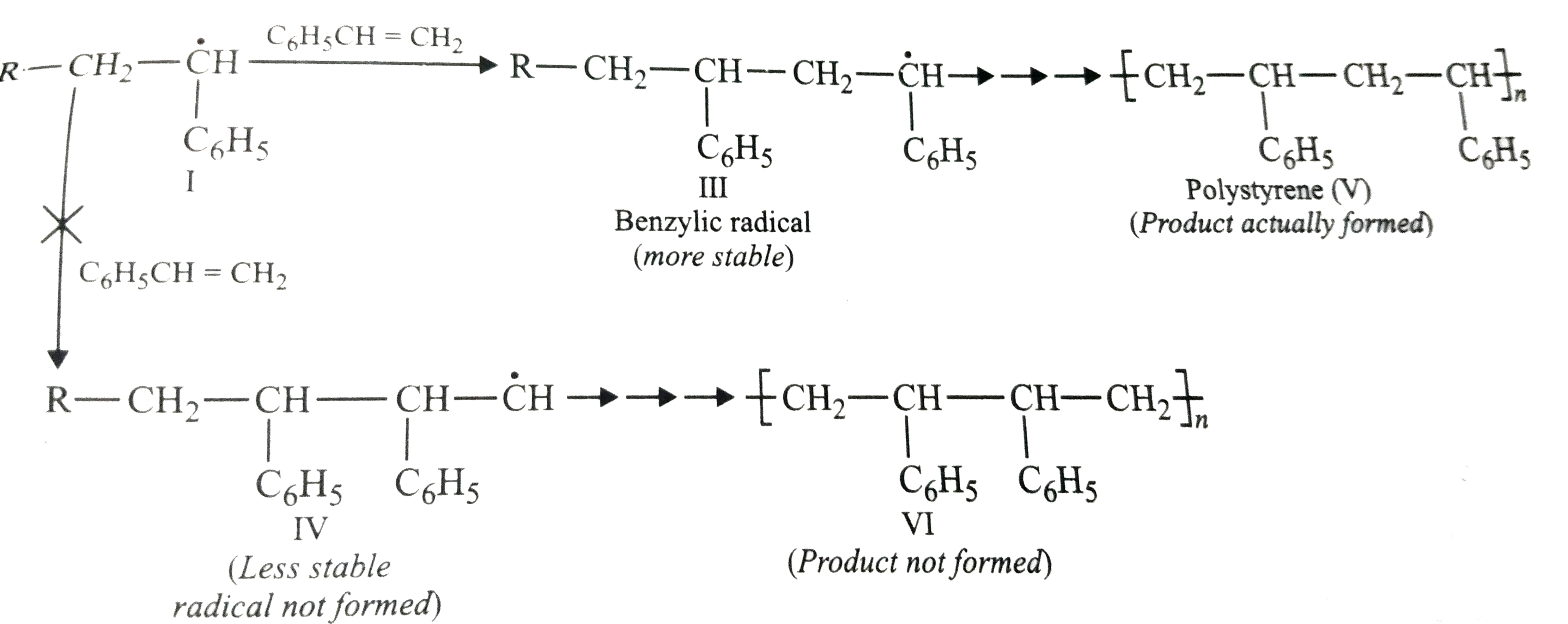
The free radical (I) then adds to another monomer molecule again giving a more stable free radical (III) rather than the less stable free radical (IV). This process continues to give ultimately polystyrene (V) in whicyh the C6H5 groups are on alternate carbon atoms rather than the product (VI) in which the C6H5 groups are on adjacent carbon atoms.
|
Topper's Solved these Questions
- POLYMERSBook:PRADEEPChapter:POLYMERSExercise:Value Based Questions with answers
Explore 5 Videos
- POLYMERSBook:PRADEEPChapter:POLYMERSExercise:Competition Focus (JEE (main and Advanced) /Medical Entrance Special) (I. Multiple Choice Questions) (with one Correct Answer)
Explore 44 Videos
- POLYMERSBook:PRADEEPChapter:POLYMERSExercise:Additional Questions (LONG ANSWER QUESTIONS)
Explore 3 Videos
- P-BLOCK ELEMENTS Book:PRADEEPChapter:P-BLOCK ELEMENTS Exercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 25 Videos
- REDOX REACTIONS Book:PRADEEPChapter:REDOX REACTIONS Exercise:Assertion reason type question
Explore 16 Videos
Similar Questions
Knowledge Check
The compound in which the distance between the two adjacent carbon atoms is largest is :
AbenzeneBetheneCbutaneDethyneIn which among the following molecules is the distance between two adjacent carbon atoms largest
ABenzeneBEthaneCEtheneDEthyeneIn which of the following , the bond length between carbon and carbon atom is equal
A2-buteneBBenzeneC1-buteneD1-propyne
The central carbon atom of a free radical contains
View SolutionThe carbon atom of carbonyl group is
View SolutionMolecule in which the distance between two adjacent carbon atoms is longest in
View SolutionThe compound in which the distance between the two adjacent carbon atoms is largest is :
View SolutionCarbocation is group of atoms that contains a carbon atom bearing only-electrons
View Solution

