(i) [SiF6]2− is known whereas [SiCI6]2− is not.Give possible reason.
(ii) Bi(V) is a stronger oxidinsing agent than Sb(v). Explain
(iii) What happes when H3PO3 is heated ?
Step by step video & image solution for (i) [SiF_(6)]^(2-) is known whereas [SiCI_(6)]^(2-) is not.Give possible reason. (ii) Bi(V) is a stronger oxidinsing agent than Sb(v). Explain (iii) What happes when H_(3)PO_(3) is heated ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- APPENDIXBOOK - PRADEEPCHAPTER - APPENDIXEXERCISE - MODEL TEST PAPER <br> (Section B)
8 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - PRADEEPCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - IMPORTANT QUESTIONS FOR BOARD EXAMINATION
29 Videos
- BIOMOLECULES BOOK - PRADEEPCHAPTER - BIOMOLECULES EXERCISE - IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)
25 Videos
Similar Questions
A ballon is filled with hydrogen at room temperature. It will burst if pressure exceeds 0.2bar. If at I bar pressure, the gas occupies 2.27L volume, up to what volume can the balloon be expanded?
View SolutionGive the IUPAC name of the following compound
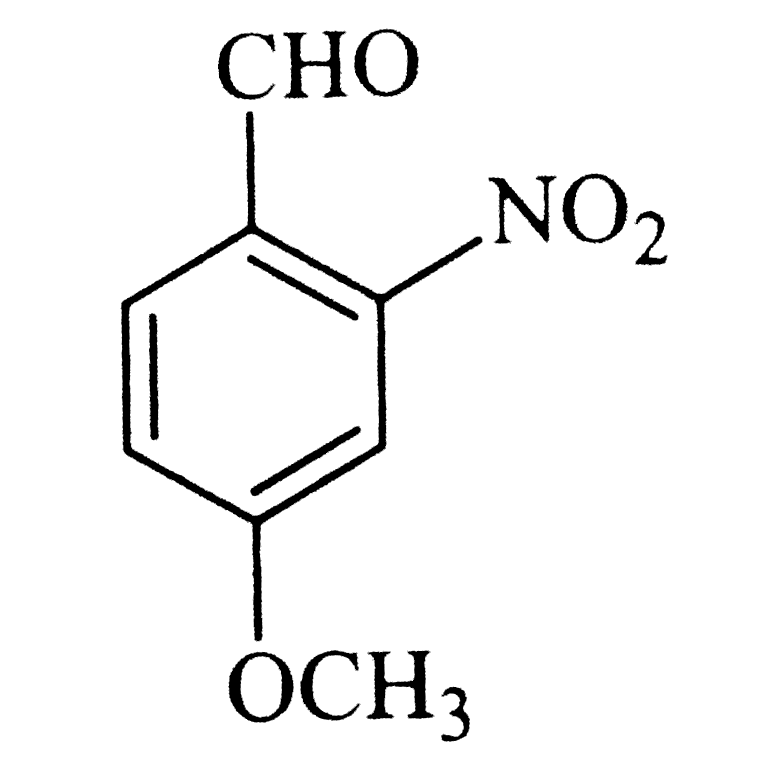
View SolutionWhat is Molisch reagent and what is it used for ?
View SolutionBi(V) and Sb(V) which may be a stronger oxidizing agent and why ?
View SolutionWhy is Bi(V) a stronger oxidant than Sb(V) ?
View Solution(a) Draw the structures of the following : (i) PCl_(5) (s) (ii) SO_(3)^(2-) Explain the following observations : (i) Ammonia has a higher boiling point than phosphin. (ii) Helium does not form any chemical compound. (iii) Bi (V) is a stronger oxidising agent than Sb (V).
View Solution(a) Account for the following observations : (i) SF_(1) is easily hydrolysed whereas SF_(6) is not easily hydrolysed. (ii) Chlorine water is a powerful bleaching agent. (iii) Bi (V) is a stronger oxidising agent than Sb(V) (b) What happens when : (i) White phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO_(2). (ii) XeF_(6) undergoes partial hydrolysis. (Give the chemical equations involved).
View SolutionWhy is Bi(V) a stronger oxidant than Sb(V) ?
View SolutionWhy is Bi(V) a stroger oxidant than Sb(V) ? Or of Bi (V) and Sb (V) which may be a stronger oxidising agent and why ?
View SolutionWhy is Bi(V) a stronger oxidant than Sb(V) ?
Or of Bi (V) and Sb(V) which may be a stronger oxidising agent and why ?
View SolutionBi(V) is a stronger oxidising agent than Sb(V) - why ?
View SolutionWhich is a stronger oxidising agent ? Sb (V), Bi (V).
View SolutionGive reasons for the following:
H3PO2 is a stronger reducing agent than H3PO3
View Solution_____out of Bi(V) and Sb(V) is stronger oxidising agent.
View SolutionWhy is Bi (V) a stronger oxidant than Sb (V)?
View SolutionWhy is Bi(V) a stronger oxidant than Sb(V) ?
View Solution

