Which of the following electrodes will act as anodes when connected to standard hydrogen electrode?

All electrodes which have negative electrode potential are stronger reducing agent than H2 gas and Fe2+/Fe(E∘=−0.44v) acts as anodes i.e option (a) and (b) are correct
Similar Questions
Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode ?
View SolutionAssertion: Standard hydrogen electrode is also called reversible electrode. Rationale: The standard hydrogen electrode acts as an anode as well as a cathode in an electrochemical cell.
View SolutionWhich of the following electrodes will act as anodes, which connected to Standard Hydrogen Electrode?
View SolutionWhich of the following electrodes will act as anodes, which connected to Standard Hydrogen Electrode ?
View SolutionAssertion:A standard hydrogen electrode is also called reversible electrode.
Reason : Standard hydrogen electrode can act both as anode as well as cathode in an electrochemical cell.
View Solutionमानक हाइड्रोजन इलेक्ट्रोड से संयोजित किए जाने पर निम्नलिखित में से कौन-से इलैक्ट्रोड, ऐनोड के रूप में कार्य करेंगे?
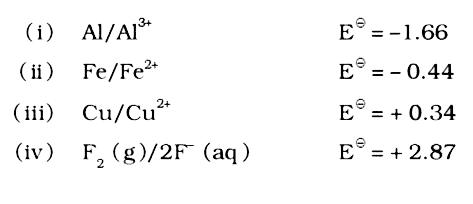
View Solutionजिंक इलेक्ट्रोड तथा मानक हाइड्रोजन इलेक्ट्रोड (SHE ) वाले सेल में जिंक इलेक्ट्रोड का कार्य करता है
View Solutionजिंक इलेक्ट्रोड तथा मानक हाइड्रोजन इलेक्ट्रोड (SHE ) वाले सेल में जिंक इलेक्ट्रोड का कार्य करता है
View Solution
