Explain the following observations:
(a) Azide ion (N3) react with 2− bromopentane thousand times faster than with neopentyl bromide in a SN2 reaction through former isa secondary halide while latter is primary.
(b) What will happen to teh stereochemistry of product of the following reaction:
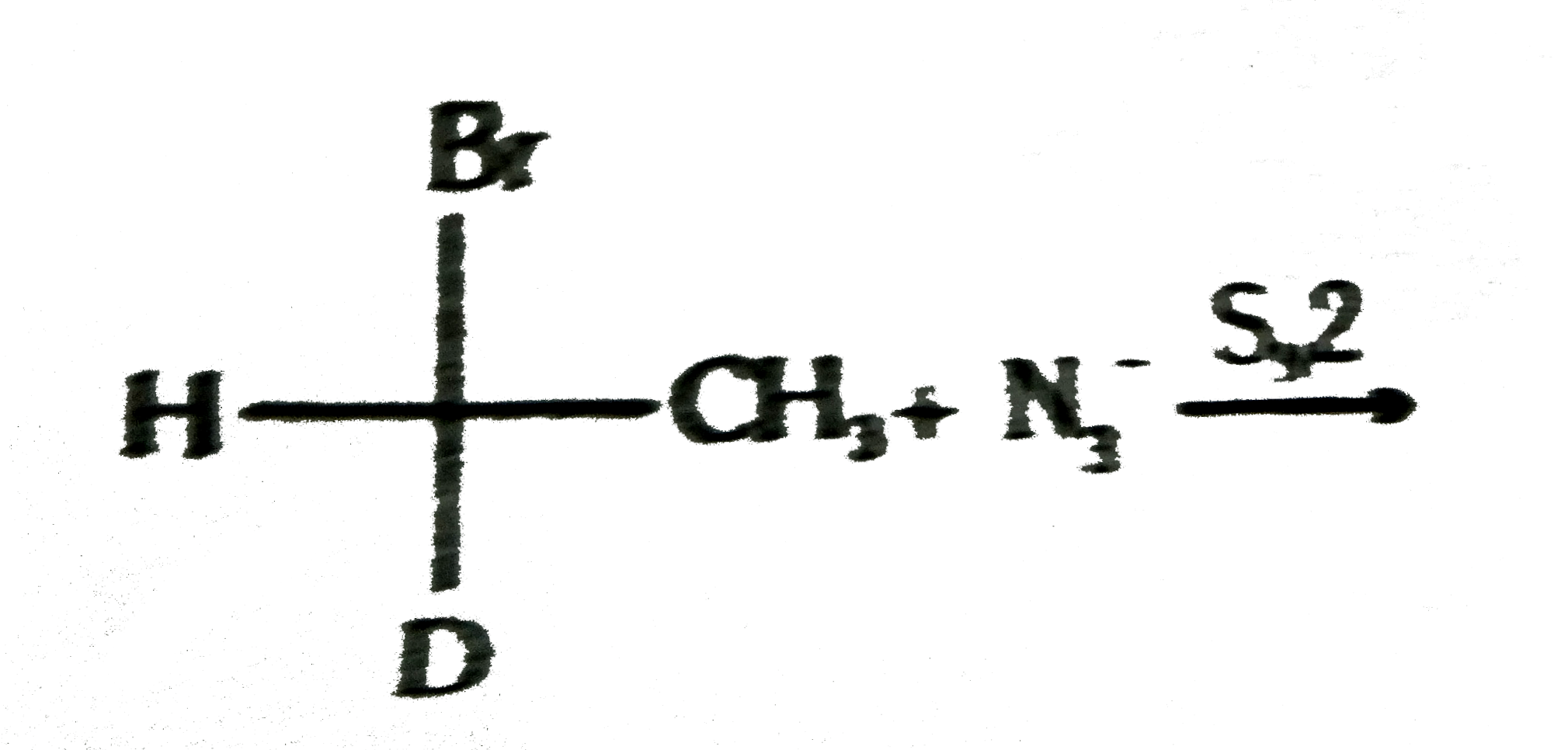
(c) What will happens to the rate if the concentration of alkyl bromide in (b) is doubled?
(d) Wha will happen to the rate if the concentration of azide ion in (b) is doubled?
(e) How the sign of optical rotation of reactant and product are related in (b)
(f) When allowed to stand in dilute H2SO4 laevo-rotatory 2-butanol slowly loss optical activity.
The correct Answer is:(a) Though neopentybromide is primary, bulky group posses very large steric hindrance to the attack fo bulky nucleophile N−3.
(##ALNCHMC08E01085_A01##)
(c) Rate will double (d) Rate will double
(e) not related (f) Racemization occur through carbocation intermediate
Step by step video, text & image solution for Explain the following observations: (a) Azide ion (N_(3)) react with 2- bromopentane thousand times faster than with neopentyl bromide in a S_(N)2 reaction through former isa secondary halide while latter is primary. (b) What will happen to teh stereochemistry of product of the following reaction: (c) What will happens to the rate if the concentration of alkyl bromide in (b) is doubled? (d) Wha will happen to the rate if the concentration of azide ion in (b) is doubled? (e) How the sign of optical rotation of reactant and product are related in (b) (f) When allowed to stand in dilute H_(2)SO_(4) laevo-rotatory 2-butanol slowly loss optical activity. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Following reaction:
(CH3)3C−Br+H2O→(CH3)3C−OH+HBr is an example of -
View Solution_E01_735_Q01.png) cannot undergoes claisen condensation.
cannot undergoes claisen condensation.
View SolutionWhat happens when sodium azide is treated with methyl bromide?
View SolutionIn SN1 reactions, rate of reaction depends on
a) Concentration of alkyl halide
b) Concentration of nucleophile
c) Nature of alkyl halide
View SolutionIn the reaction, R – Br + Cl rarr R-Cl + Br^(-) . The rates of SN_2 reaction of ethyl bromide (I), n-propyl bromide (II), isobutyl bromide (III) and neopentyl bromide (IV) follow the order:
View SolutionIn S_N1 reactions, rate of reaction depends on a) Concentration of alkyl halide b) Concentration of nucleophile c) Nature of alkyl halide
View Solutionनिम्न एल्किल ब्रोमाइड में से SN2 अभिक्रिया में सबसे कम क्रियाशील ब्रोमाइड है
View SolutionAmong the choices of alkyl bromide , the least reactive bromide in a S_(N^(2)) reaction is :
View Solution
