The standard reduction potential E∘(Bi3+/Bi) and E∘(Cu2+/Cu) are 0.226V and 0.344V respectively. A mixture of salts of bismuth and copper at unit concentration each is electrolysed at 25∘C. To what value can [Cu2+] be brought down before bismuth starts to deposit in electrolysis.
The correct Answer is:[Cu2+]=10−4M
ECu2+/Cu=E∘Cu2+/Cu+0.0592lgc[Cu2+]
[Cu2+]=10−4m
Topper's Solved these Questions
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE-04 [B]
23 Videos
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE-05 [A]
28 Videos
- ELECTROCHEMISTRYBOOK - ALLENCHAPTER - ELECTROCHEMISTRYEXERCISE - EXERCISE-03
24 Videos
- CHEMISTRY AT A GLANCEBOOK - ALLENCHAPTER - CHEMISTRY AT A GLANCEEXERCISE - ORGANIC CHEMISTRY
483 Videos
- HYDROCARBONBOOK - ALLENCHAPTER - HYDROCARBONEXERCISE - MCQ
15 Videos
Similar Questions
The e.m.f. of cell: H2(g) |Buffer| Normal calomal electrode is 0.6885V at 40∘C when the barometric pressure is 725mm of Hg. What is the pH of the solution E∘calomal=0.28.
View SolutionWhen, during electrolysis of a solution of AgNO39650 colombs of charge pass through the electroplating path, the mass of silver deposited on the cathode will be:
View SolutionMatch the items of column I to those of column I:
Column−IColumn−II(A)Cell constant(p)E0cathode+E0anode(B)Anode(q)l/A(C)Conductance(r)Mass of product by 1 coulomb of electricity(D)Electrochemical equivalent(s)(Resistance)−1(E)E0cell(u)Involve oxidation
View SolutionIUPAC Name of following compound is :
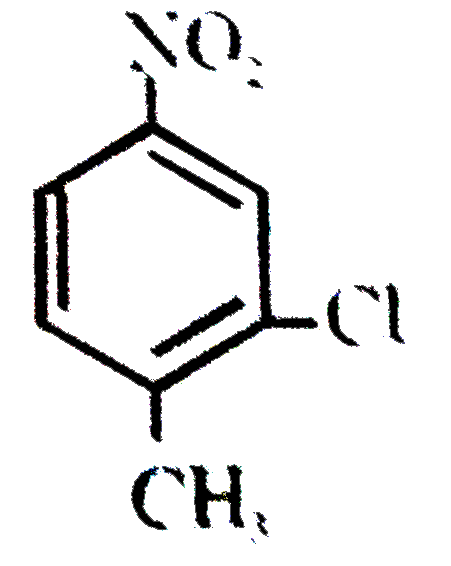
View Solution
View SolutionThe standard reduction potential of E_(Bi^(3+)//Bi)^(@) and E_(Cu^(2+)//Cu)^(@) are 0.226 V and 0.344 V respectively. A mixture of salts of Bi and Cu unit concentration each is electrolysed at 25^(@)C . To what value can [Cu^(2+)] be brought down before bismuth starts to deposit during electrolysis ?
View SolutionThe standard reduction potential of Cu^(2+)//Cu and Cu^(2+)//Cu^(+) are 0.337 and 0.153 respectively. The standard electrode potential of Cu^(+)//Cu half - cell is
View SolutionThe standard reduction potentials of Cu2+/Cu and Cu2+/Cu+ are 0.337 V and 0.153V respectively. The standard electrode potential of Cu+/Cu half-cell is
View SolutionThe Standard reduction potential values, E^(@)(Bi^(3+)//Bi) and E^(@)(Cu^(2+)//Cu) are 0.226 V and 0.344 V respectively. A mxiture of salt of bismut and copper at unit concentration each is electrolysed at 25^(@)C to what value can [Cu^(2+)] be brought down before bismuth starts to deposit, in electrolysis.
View SolutionThe standard reduction potentials of Cu^(2+)|"Cu and"Cu^(2+)| Cu^(+) are 0.337V and 0.153V respectively The standard electrode potential of Cu^(+)|Cu half cell is
View SolutionThe Standard reduction potential values, E∘(Bi3+/Bi) and E∘(Cu2+/Cu) are 0.226 V and 0.344 V respectively. A mxiture of salt of bismut and copper at unit concentration each is electrolysed at 25∘C to what value can [Cu2+] be brought down before bismuth starts to deposit, in electrolysis.
View SolutionAt 25∘C, the reduction potential of Cu2+∣Cu half - cell is 0.28V. If E∘ of Cu2∣Cu half-cell is +0.34V, find the molar concentration of Cu2+ion in the half - cell.
View SolutionGiven that the standard potentials ((E^(@))) of CU^(2+)//Cu and CU^(+)//Cu are 0.34V and 0.522V respectively , the E^(@) of CU^(2+)//CU^(+) is :
View SolutionCu2+/Cu तथा Cu2+/Cu+ के लिए मानक अपचयन विभव के मान क्रमश: 0.337 V तथा 0.153 V हैं | अर्द्ध-सेल, Cu+/Cu के लिए मानक इलेक्ट्रोड विभव का मान होगा
View SolutionThe standard reduction potentials of Cu^(2+)//Cu and Cu^(2+)//Cu^(+) and 0.337 and 0.153 V respectively. The standard electrons potential of Cu^(+)//Cu half cell is
View Solution

