Azoetropes are constant boiling mixtures, which like a pure chemical compound boils at a constant temperature and distills over completely at same temperature without change in composition. There may be two type of binary solution which gives azeotropic mixture.
Type-I: Mixture showing minimum in "Boiling point-Composition curve".
Type-II: Mixture which showns a maximum in "Boiling point-Composition curve" Both types are shown below:
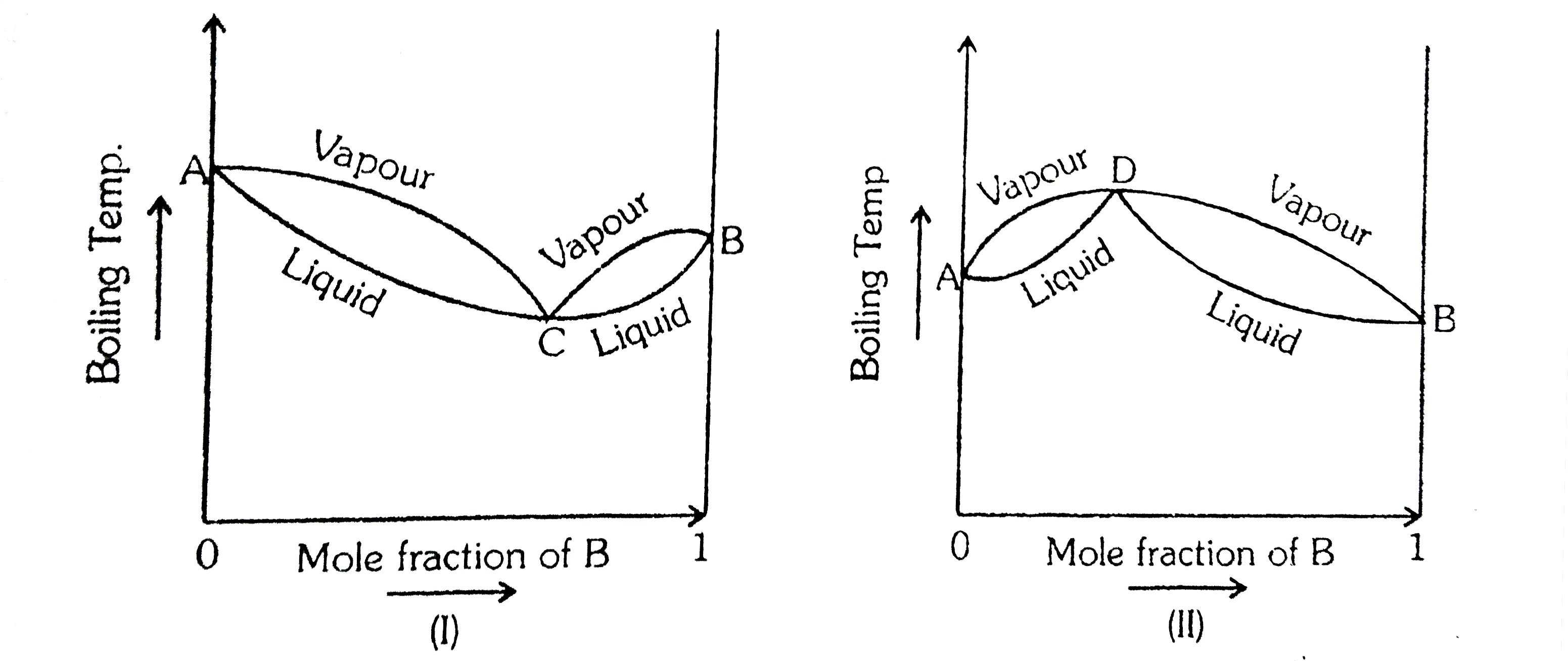
Let the solution of "water-ethanol" corresponds to Type-I and at point 'C", it had a composition of 95.6% of by weight in vapour phase". if this solution is boiled at 78.13∘C then find out the % by wt.of water in "liquid phase" (Given TC(temperature at point C) =78.13∘C:-
A
95.6%
B
4.4%
C
72.4%
D
63%
Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Azoetropes are constant boiling mixtures, which like a pure chemical compound boils at a constant temperature and distills over completely at same temperature without change in composition. There may be two type of binary solution which gives azeotropic mixture.
Type-I: Mixture showing minimum in "Boiling point-Composition curve".
Type-II: Mixture which showns a maximum in "Boiling point-Composition curve" Both types are shown below:
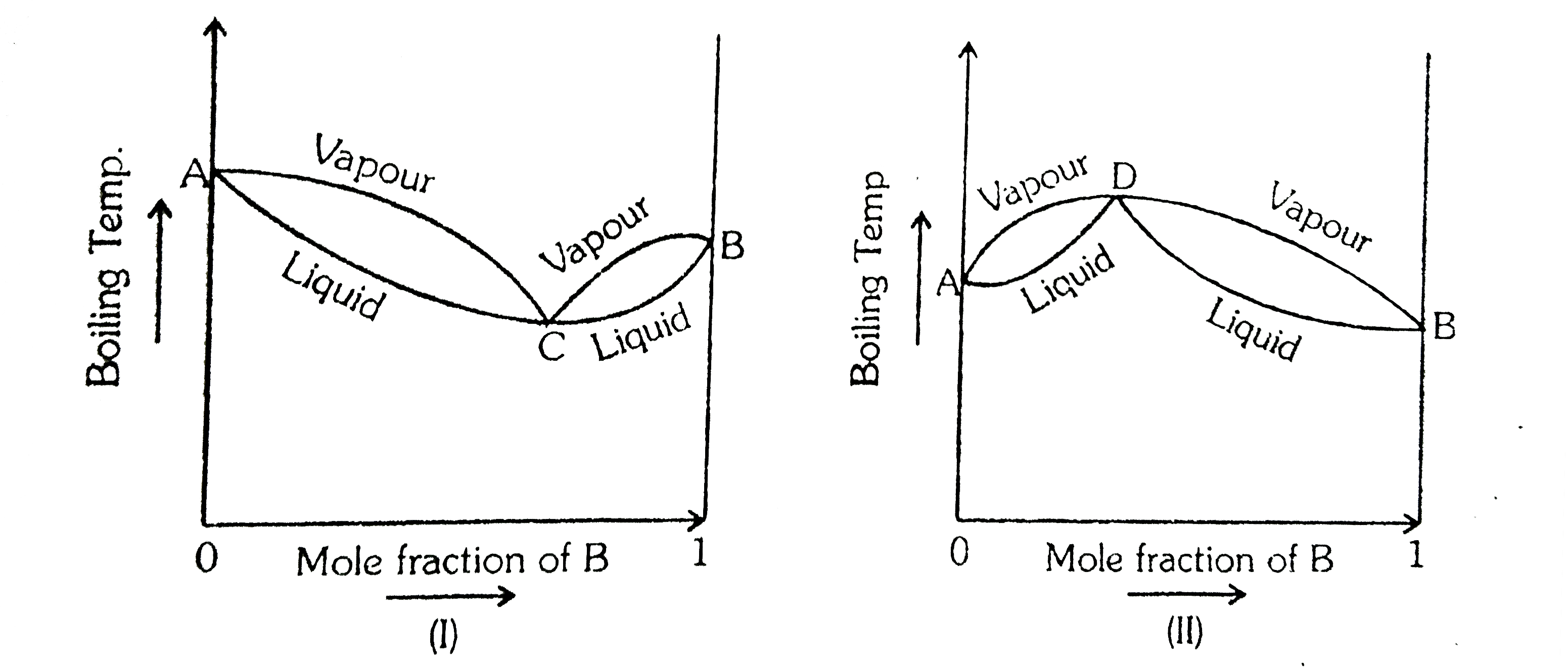
For figure (I) and (II) predict which of the component(i.e., A and B) is more volatile?ALiquid ABLiquid BCBoth have equal volatilityDCan't predictAzoetropes are constant boiling mixtures, which like a pure chemical compound boils at a constant temperature and distills over completely at same temperature without change in composition. There may be two type of binary solution which gives azeotropic mixture.
Type-I: Mixture showing minimum in "Boiling point-Composition curve".
Type-II: Mixture which showns a maximum in "Boiling point-Composition curve" Both types are shown below:
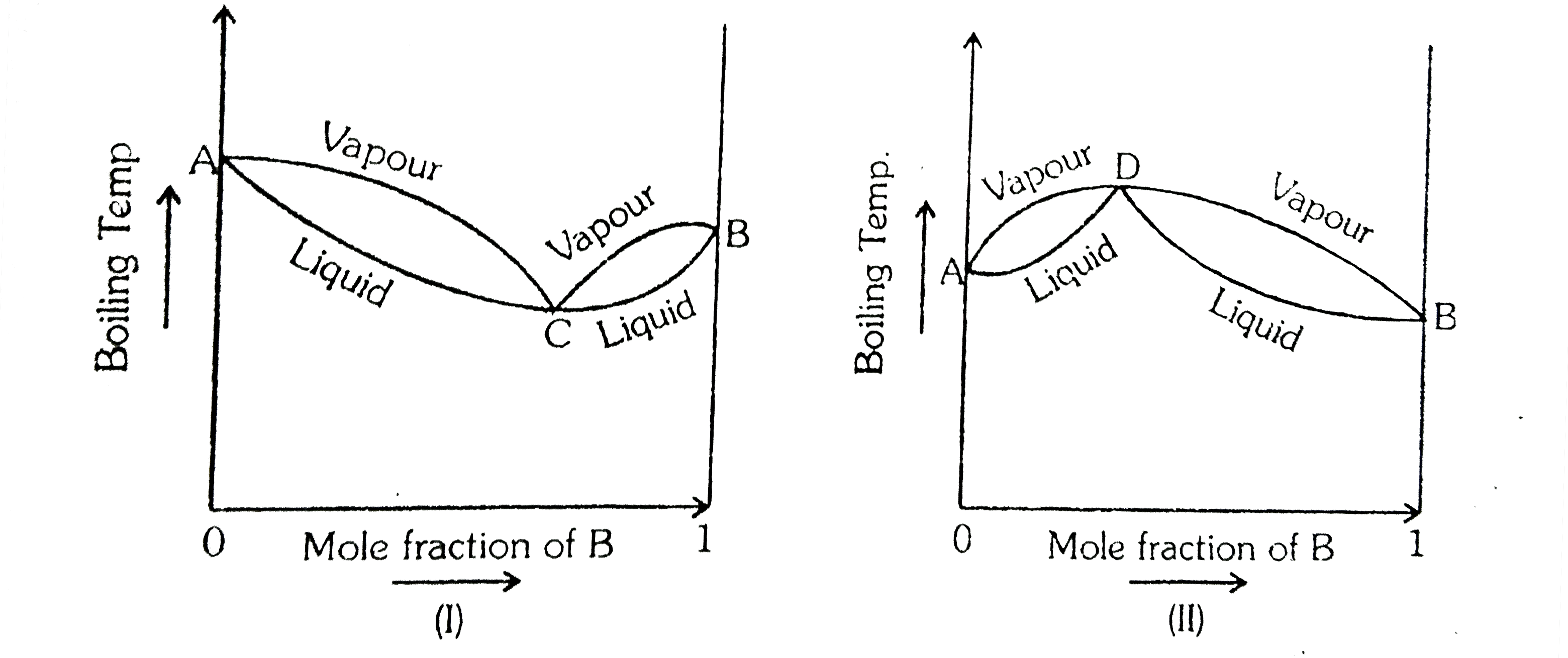
Which of the type above (i.e., Type or Type-II) shows negative deviation from Raoul's law?ASolution of Tupe-IBSolution of Tupe-IICBoth type-I and Type-IIDNone of the aboveConstant boiling mixtures are called :
AIdeal soluteBAzeotropesCIsotonicDZeotropes
The mixture that forms maximum boiling azeotrope is
View SolutionWhich of the following is low boiling azeotropic mixture :-
View SolutionSolution distilled without change in composition at a temperature is called
View SolutionThe mixture that forms maximum boiling azeotrope is :
View SolutionThe mixture that forms maximum boiling azeotrope is :
View Solution

