For an orbital the graph between ψ(r) and r (distance from nucles) is :
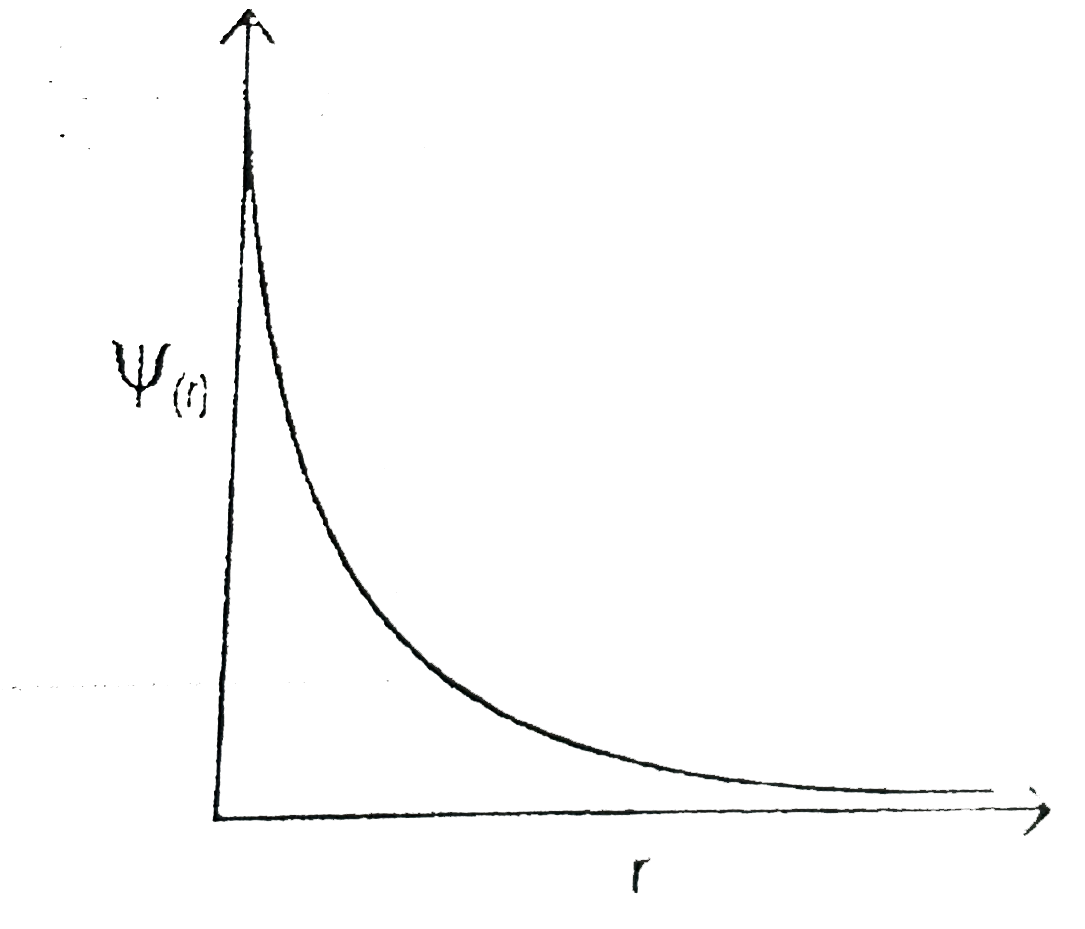
Find the value of n+l of the orbital.
A
1
B
2
C
3
D
None of these
Video Solution
Text Solution
The correct Answer is:A
For an orbital the …………..
It is 1s orbital.
|
Similar Questions
Knowledge Check
In an orbital electrons are filled according to Aufbau principle, Pauli's exclusion principle and Hund's rule of maximum muliplicity .
According to Aufbau principal the orbital are filled in order to their increasing energies . In the order of increase of energy of orbitals cna be calculated from (n+l) rule. Lower the value of (n+l) for an orbital the lowest energy hence orbital are filled in order of increasing (n+l) value. If two orbitals have same (n+l) value, the orbital with lower value of 'n' has lower energy hence it is filled first.
According to Pauli's exclusion principle, an orbital can have maximum two electrons with opposite spin.
According to Hunds rule pairing of electron in degenerate orbitals of the same sub shell does not take place until each orbital belonging to that sub shell has got one electron each i.e., singly occupied.
If n=4, how many element are possible?A20B14C32D16When a planet is orbiting around the sun in an elliptical orbit, r1andr2 denote its closest and farthest distances from the sun. Then the ratio of the magnitudes of maximum and minimum gravitational forces between them is
Ar1:r2Br21:r22Cr2:r1Dr22:r21Critical velocity of a satellite orbiting around the earth (radius R) at a distance of 8 R from the surace is 2.5 km/s. Critical velocity of another satellite orbiting at a height of 15 R from the surface is
A1.40 km/sB1.87 km/sC4.44 km/sD3.33 km/s
What are the values of n and l for 4d orbitals ?
View Solution" The "(n+l)" value of "3d" orbital is "
View SolutionWhat are the value of n, l and m for 2p-orbitals ?
View SolutionValue of n= 5 & value of ml = +2 . Find the number of orbitals.
View SolutionThe graph for an orbital between |φ|2 and r(radial distance) is shown below. The sum of principle quantum number, azimuthal, quantum number and magnetic quantum number of this orbital is ____________.
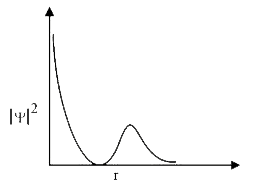
View Solution

