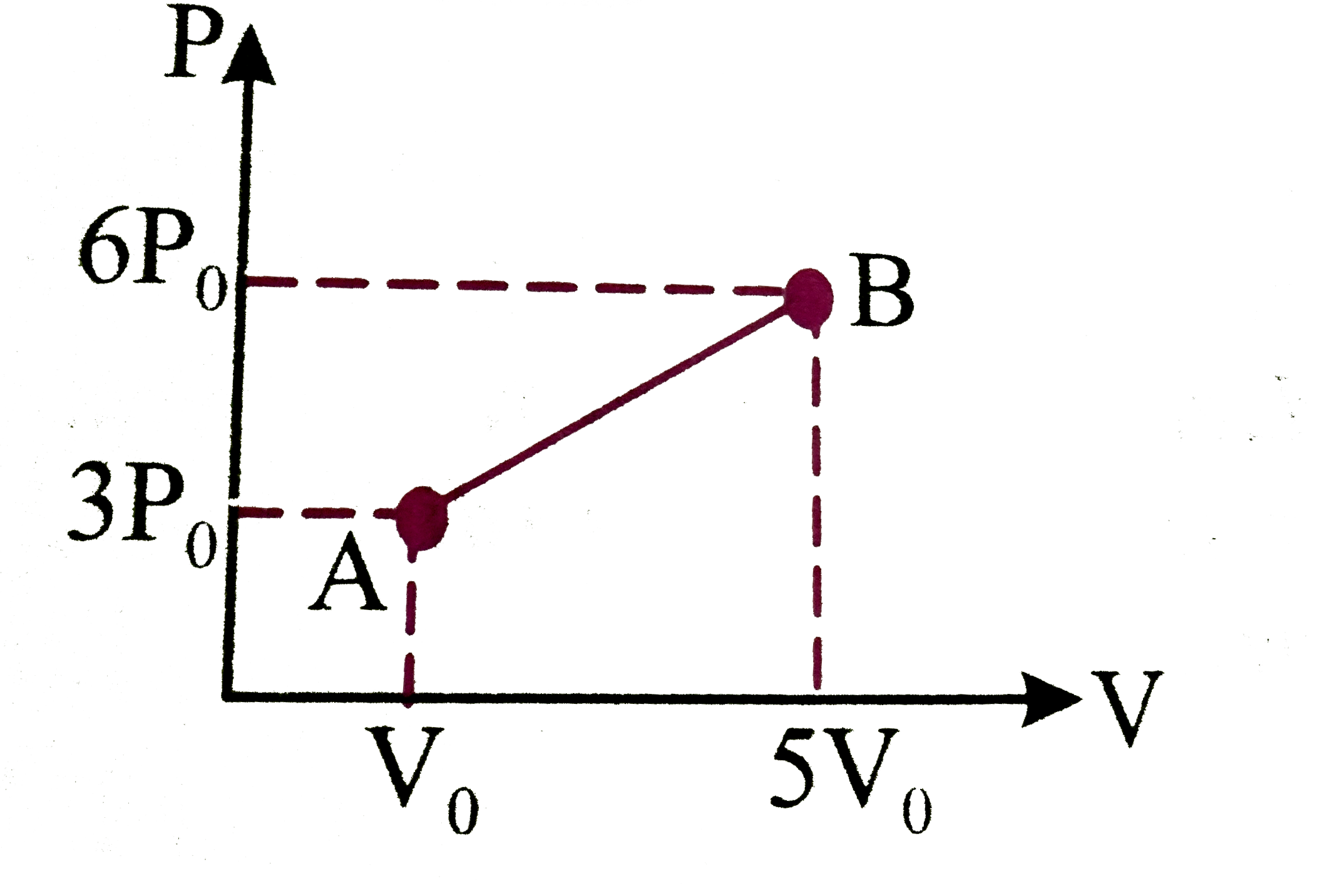
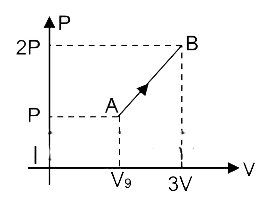
1 mol of an ideal gas undergoes different thermodynamical process in P−V diragram given below :
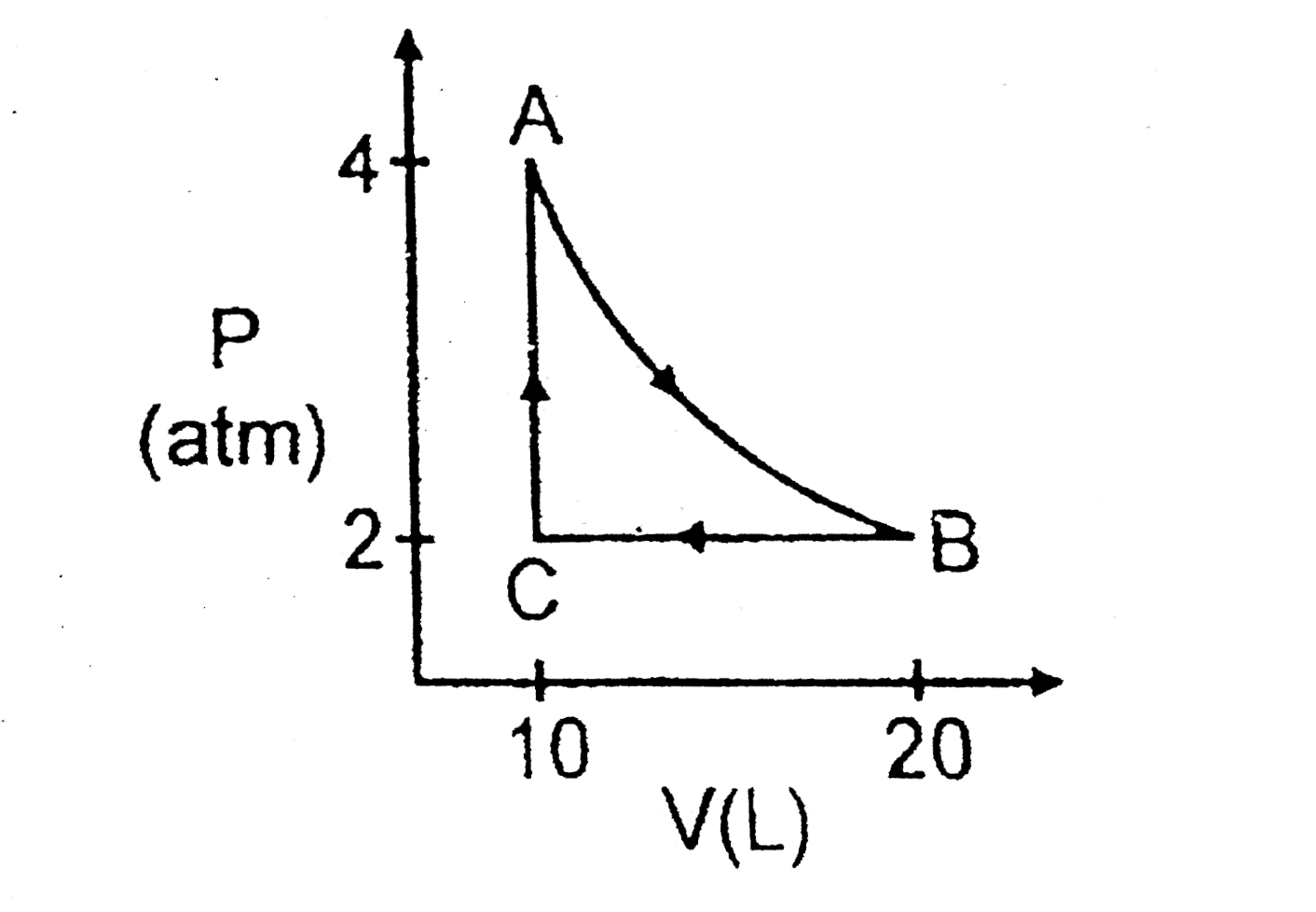
What will be the value of ΔH and ΔE for overall process ?
A
ΔH=0
B
ΔE=0
C
ΔH=48.4 calorie
D
ΔE=13.82 calorie
Video Solution
Text Solution
The correct Answer is:A, B
Overall process is cyclic so ΔE=ΔH=0
|
Similar Questions
Knowledge Check
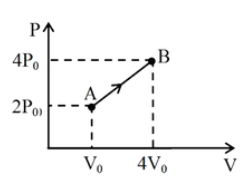
1 mol of an ideal gas undergoes different thermodynamical process in P−V digram given below :
If temperature at point C is TK then :ATemperature at point A is TK.BTemperature at point A is 2TK.CTemperature at point B is TK.DTemperature at point B is 2TK.An ideal gas undergoes the cyclic process shown in a graph below :
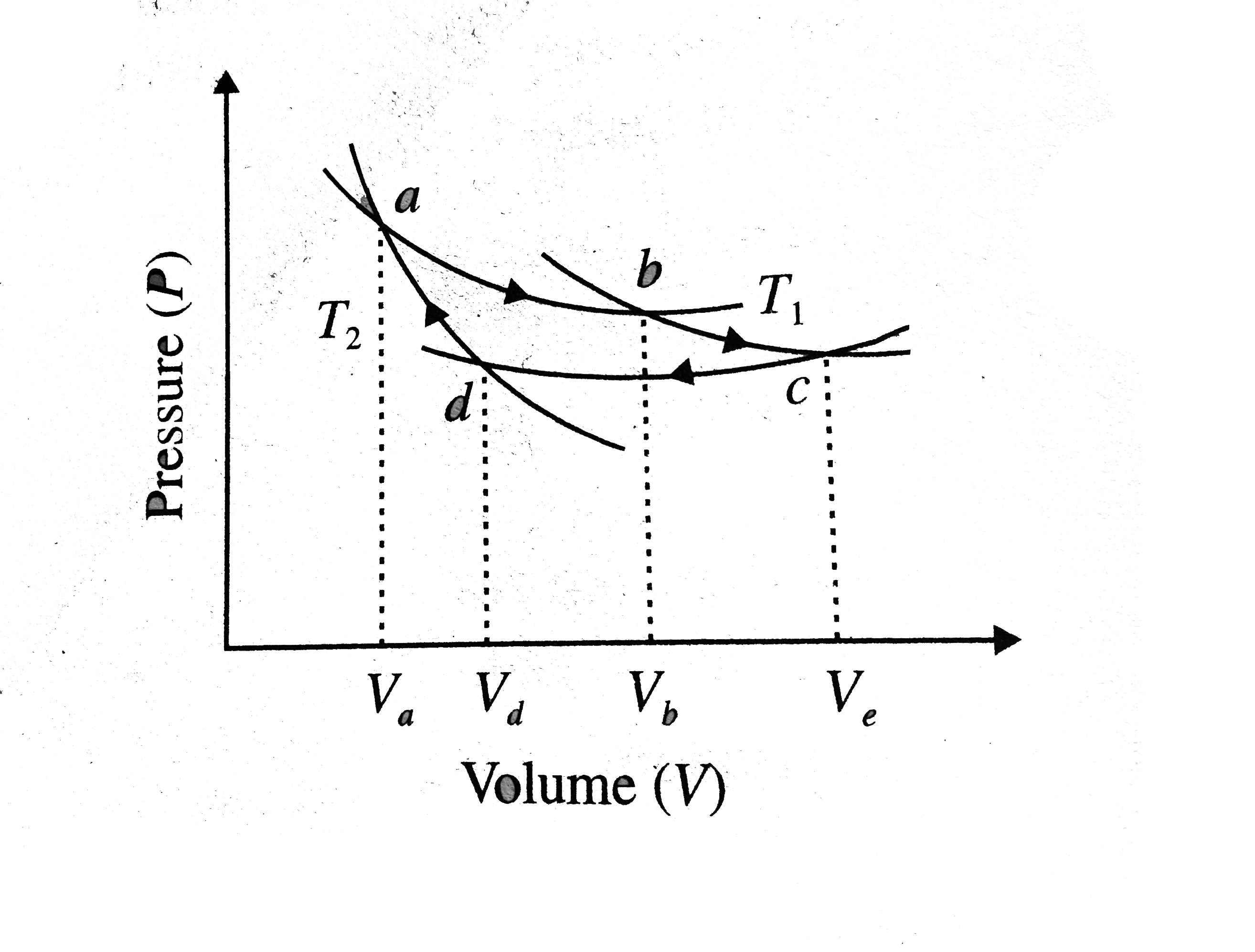 AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVd
AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVdOne mole of a monoatomic ideal gas undergoes a thermodynamic process such thatV3T2= constant. Then,
Awork done by the gas is 200R when temperature of the gas is raised by 300 KBthe specific heat of the gas in the process is 136RCwork done by the gas is 300R when temperature of the gas maised by 600 KDthe specific heat of the gas in the process is 214 R
During the thermodynamic process shown in figure for an ideal gas
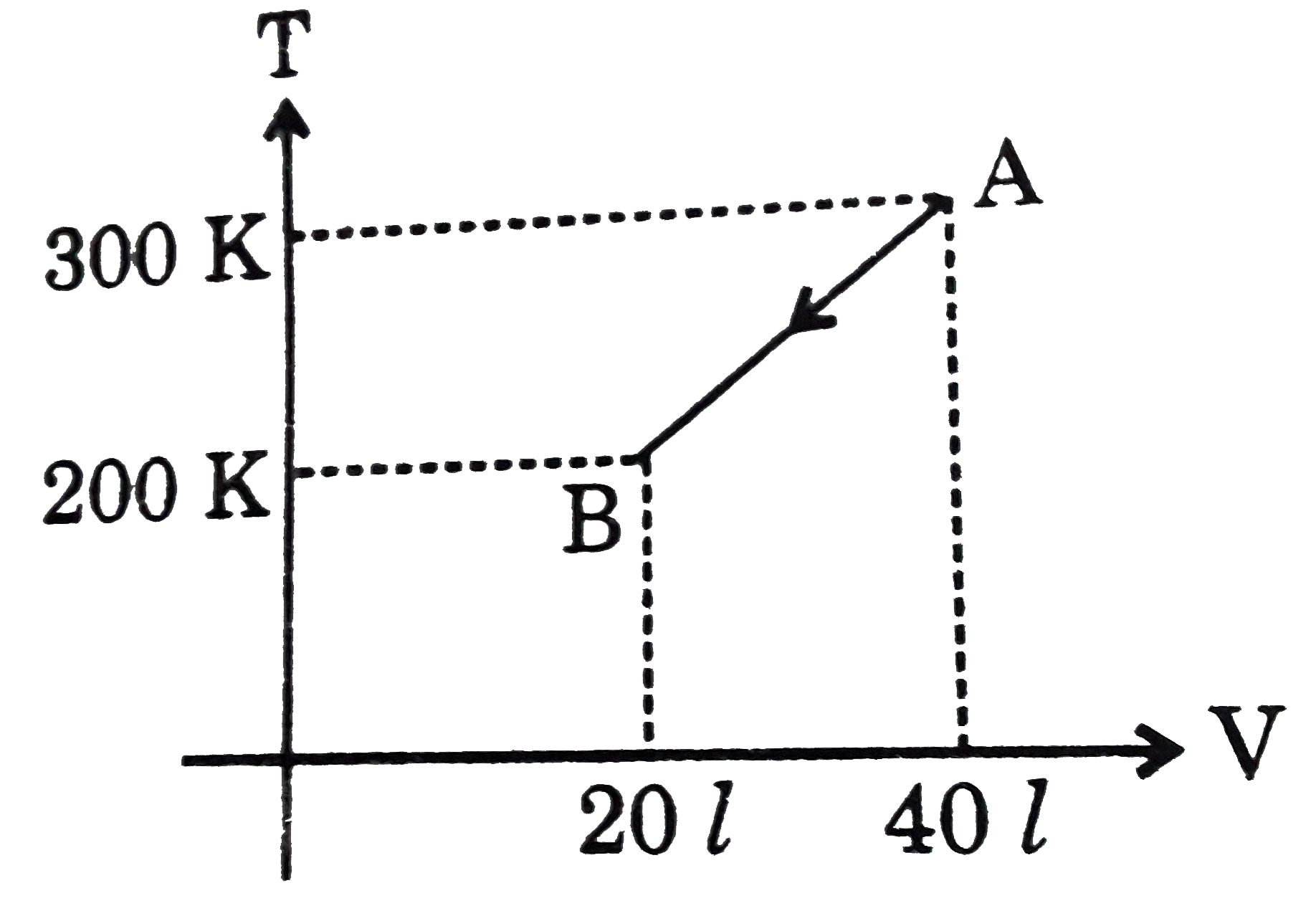
View SolutionFor 1 mol of an ideal gas work in process AB will be:
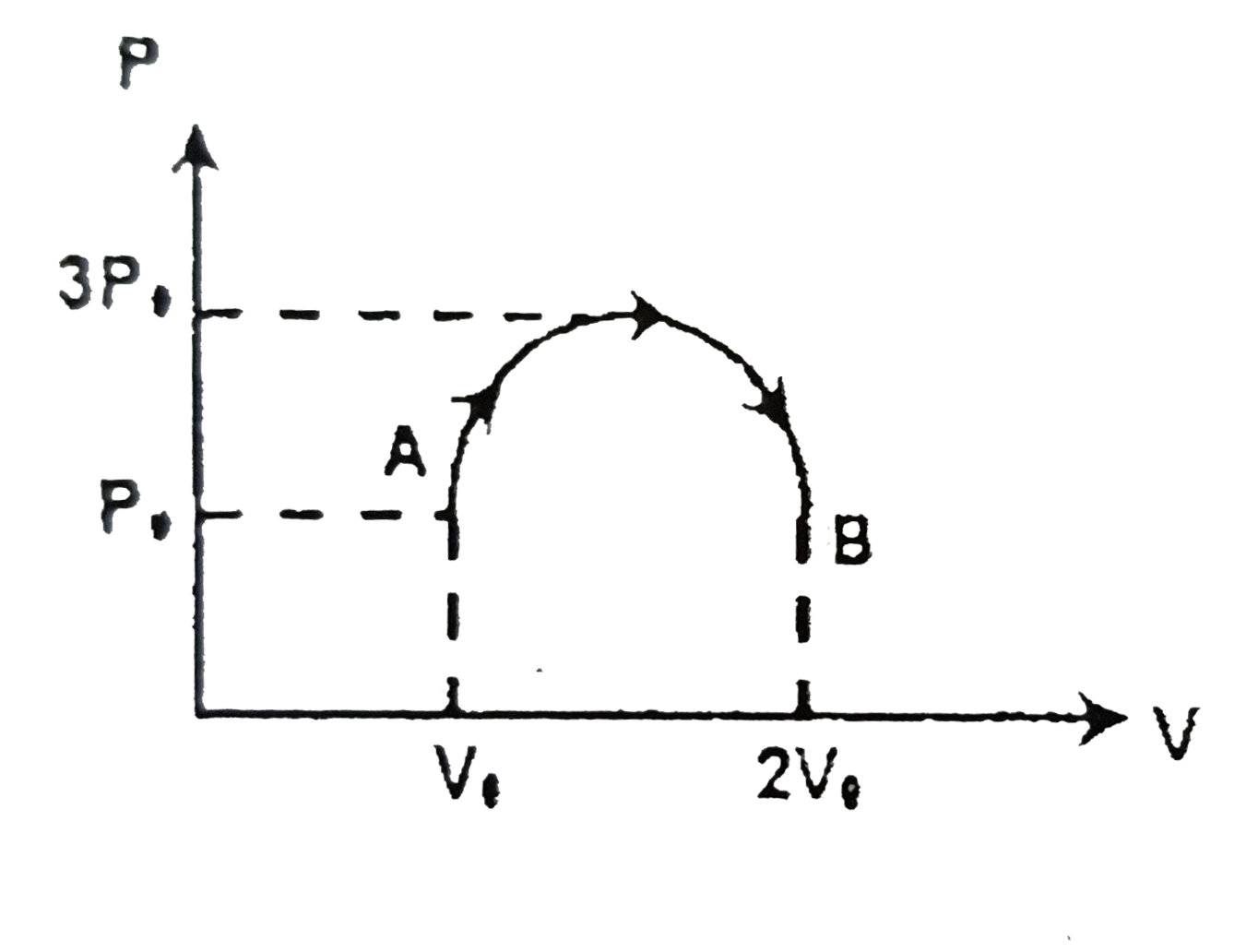
View SolutionOne mole of an ideal monoatomic gas is taken through the thermodynamic process shown in the P−V diagram. The heat supplied to the system is
View SolutionIn a given process on an ideal gas, dW=0anddQ<0. Then for the gas
View SolutionOne mole of a monoatomic ideal gas undergoes process AB in given P – V diagram then average specific heat for this process is:
View Solution