Identification of +m & −m groups : If the first atom of the group has lone pair or negative charge shows +m effect. +m group increases electron density at ortho and para position of benzene ring. If the group has vacant p−orbital on first atom, also a multipal bonded group in which second atom is more electronegative than the first then it shows −m effect. −m group decreases electron density of ortho and para position of benzene ring.
Which of the following compound/s have more electron density than
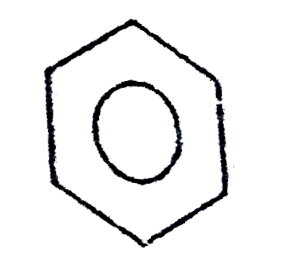
A
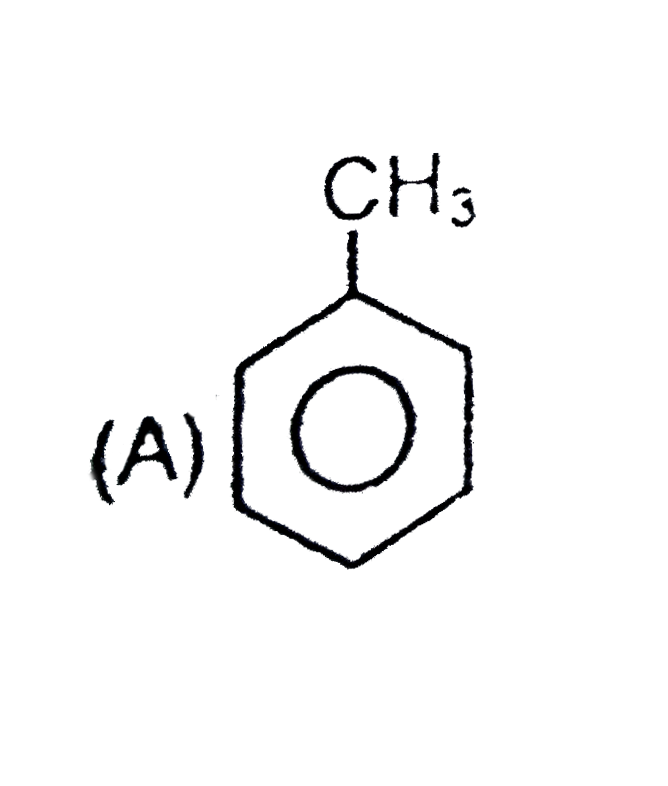
B
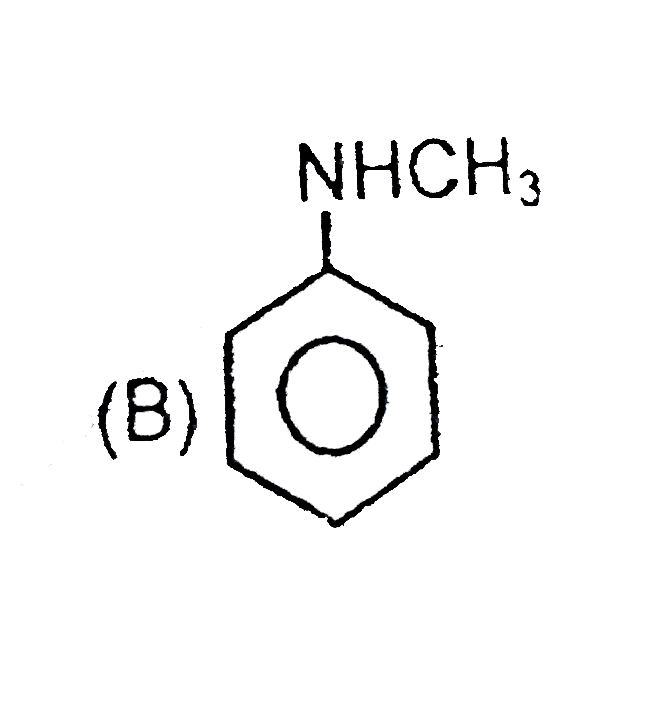
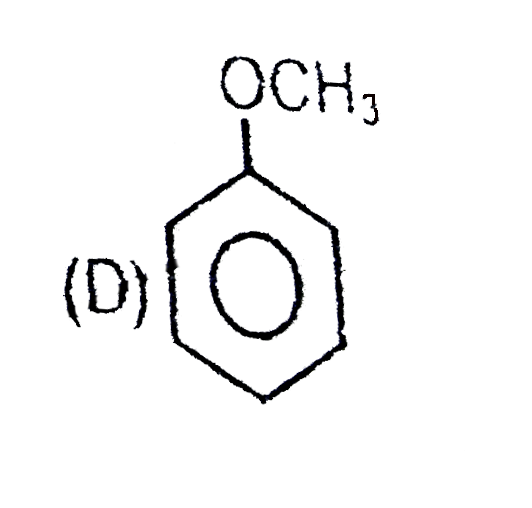
C
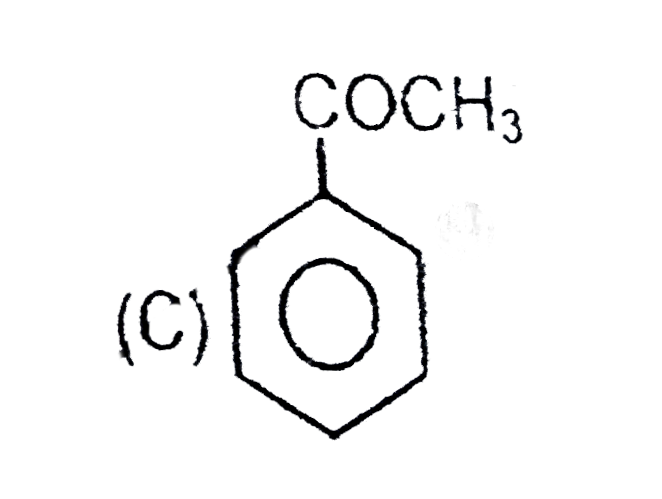
D
Video Solution
Text Solution
The correct Answer is:A, B, D
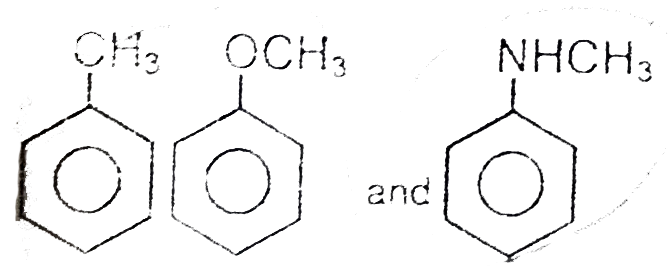 have more +m effect
have more +m effect
than 
|
Similar Questions
Knowledge Check
Identification of +m & −m groups : If the first atom of the group has lone pair or negative charge shows +m effect. +m group increases electron density at ortho and para position of benzene ring. If the group has vacant p−orbital on first atom, also a multipal bonded group in which second atom is more electronegative than the first then it shows −m effect. −m group decreases electron density of ortho and para position of benzene ring.
Identify which of following group/s show −m effect when attached with benzene ring.A−NH2B−OɵC−O∣∣C−O∣∣C−CH3D−O∣∣C−NH−CH3Which of the following group show +M and −l effect?
A−C∣∣O−FB−C∣∣O−ORC−OΘD−OHWhich of the following group show −m and −I effect?
A−NO2B−NH2C−OHD−Cl
Which of the following group show −m effect?
View SolutionIdentify which of the following group as +M as well as −M?
View SolutionWhich group show –M effect ?
View SolutionWhich of the following group shows +m effects?
View SolutionAssertion: Phenol is more reactive than benzene towards electrophilic reactions.
Reason : The +R effect of OH group increases the electron density on benzene nucleus.
View Solution
