Which pair of two isomeric complexes is difficult to differentiate by measuring molar electrical conductivity ?
A
[Co(NH3)5SO4]Cl,[Co(NH3)5Cl]SO4
B
[Co(H2O)6Cl3],[Co(H2O)4Cl2]Cl.2H2O
C
[Pt(NH3)3NO2]Cl,[Pt(NH3)3ONO]Cl
D
[Pt(NH3)4Cl2]Br2,[Pt(NH3)6]Cl4
Text Solution
The correct Answer is:C
(c ) both produce 2 ions.
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - D.VBT, CFT, Hybridisation
122 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - E. Stability, Colour and Magnetic Properties of Complexes
60 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - B.Nomenclature of Coordination Compounds
27 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS BOOK - OP TANDONCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS EXERCISE - INTEGER
2 Videos
- ELECTROCHEMISTRYBOOK - OP TANDONCHAPTER - ELECTROCHEMISTRYEXERCISE - Matrix-Matching Type Question
3 Videos
Similar Questions
Knowledge Check
Which of the following Werner's complex has least electrical conductivity ?
A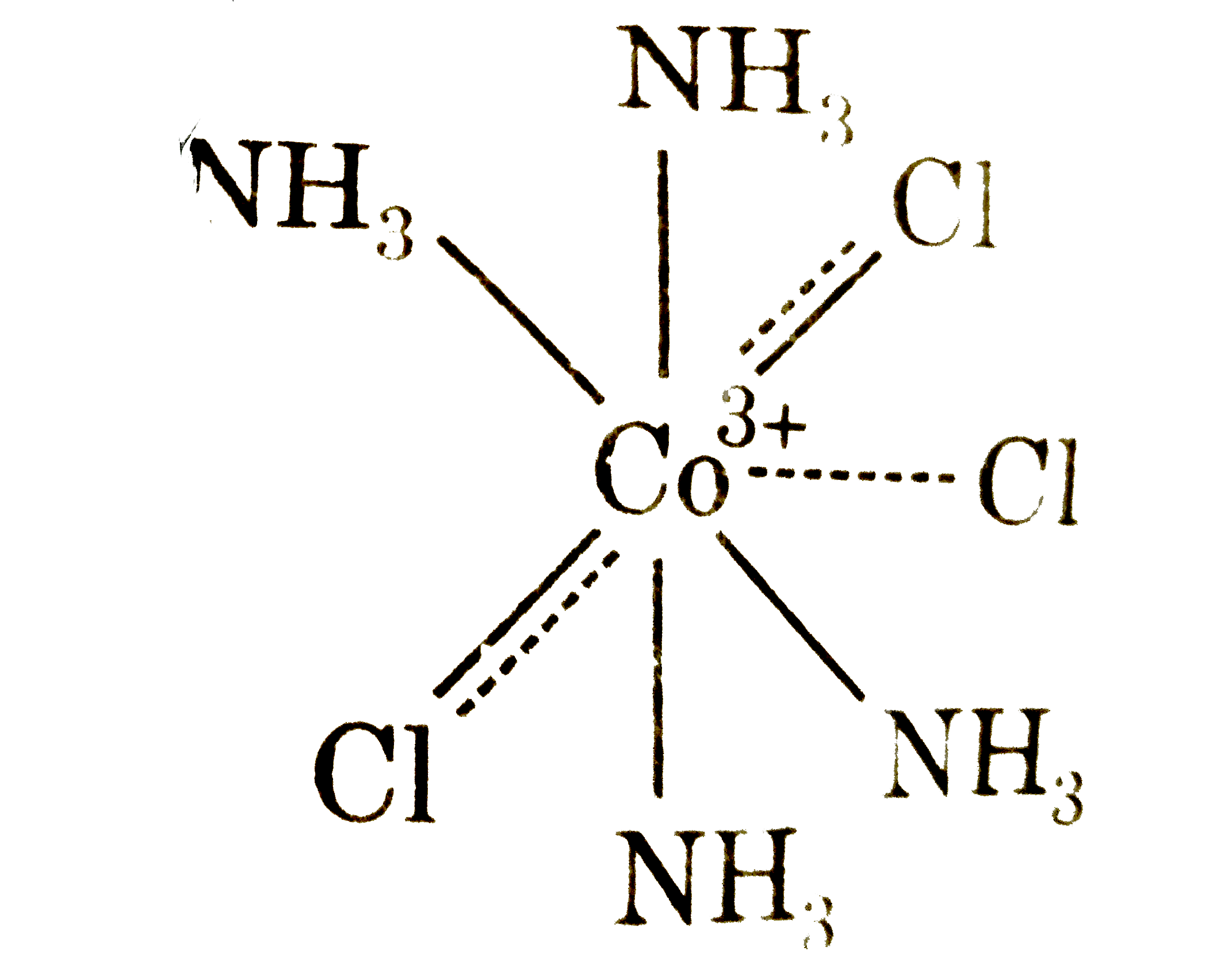 B
B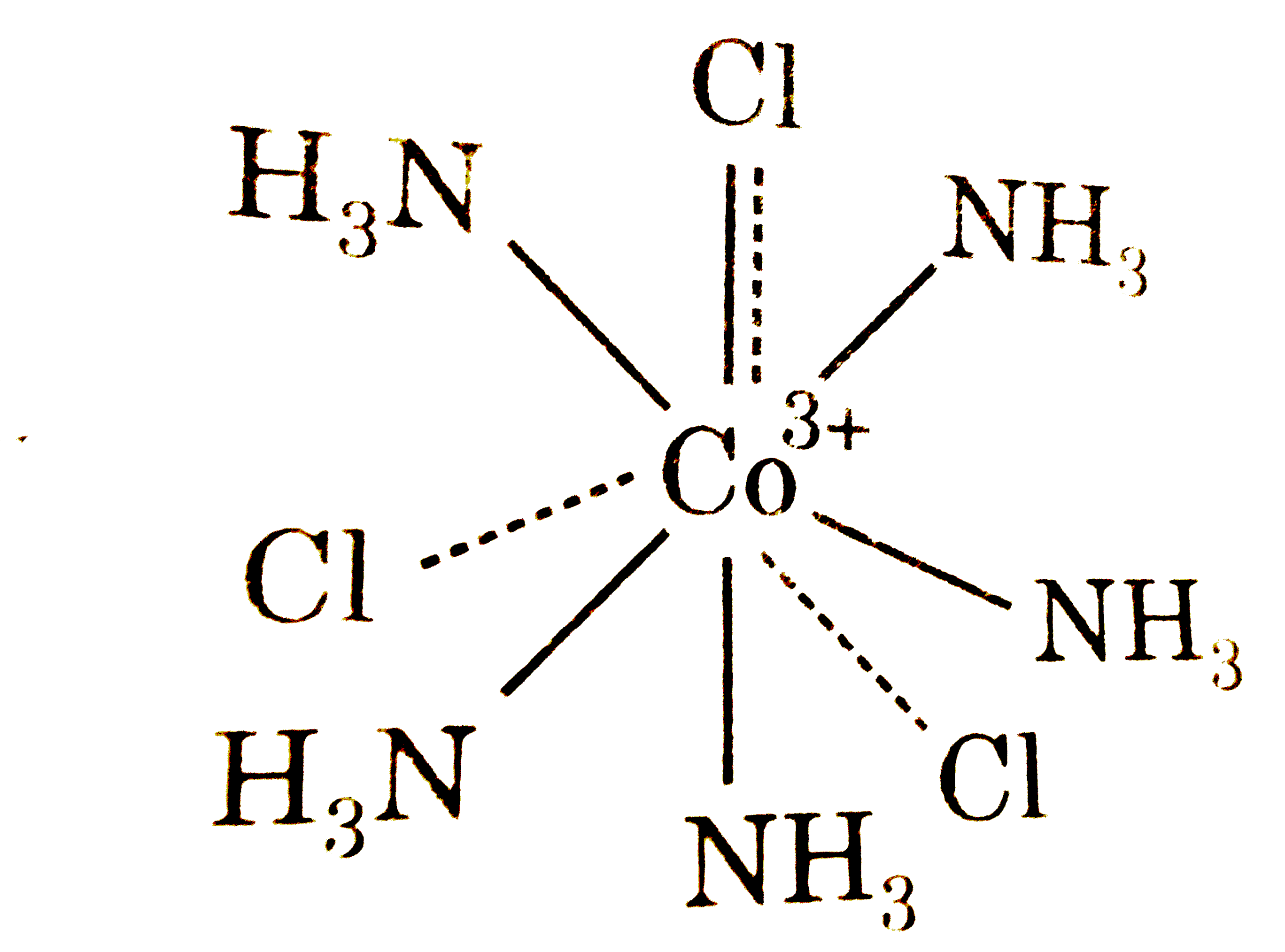 C
C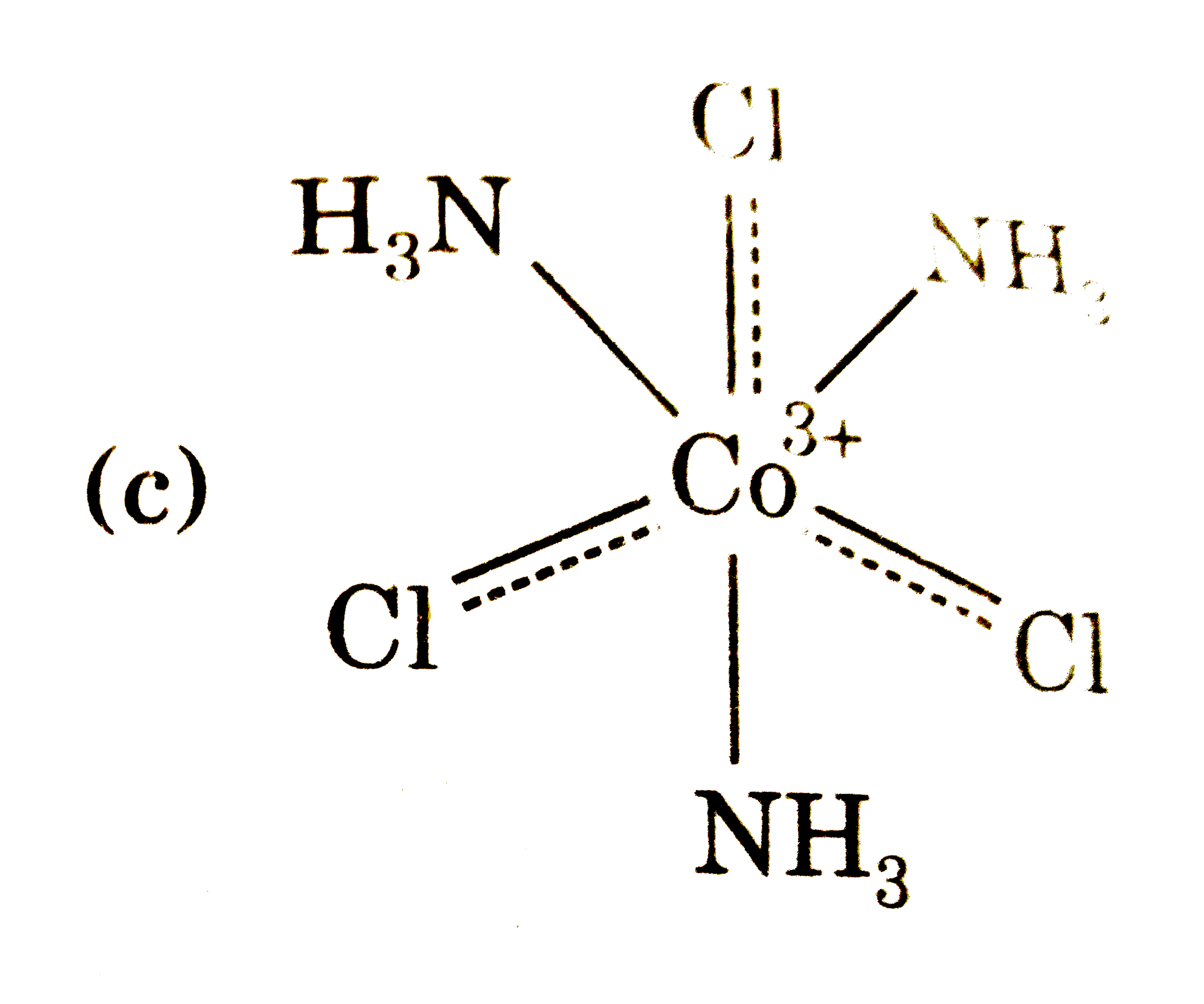 D
D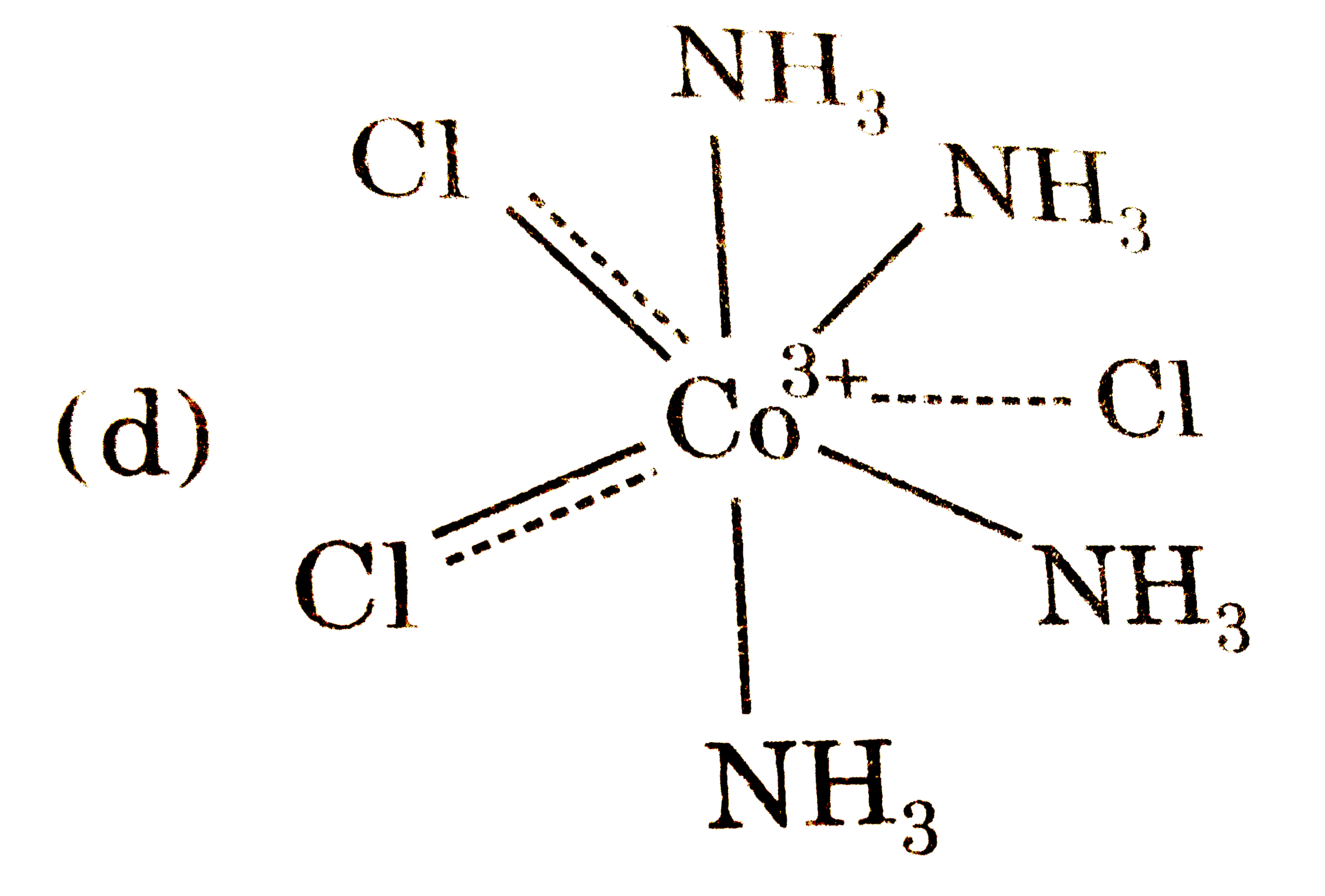
Which of the following complexes ionization isomerism ?
A[Cr(NH3)6]Cl3B[Cr(en)2]Cl2C[Cr(en)3]Cl3D[Co(NH3)5Br]SO4Which complexes show geometrical isomerism ?
ATetrahedralBOctahedralCSquare planarDLinear
Which of the following complexes has lowest molar conductance
View SolutionWhich of the following isomeric pairs shows ionization isomerism?
View SolutionWhich of the following complex has lowest molar conductivity ?
View SolutionWhich of the following is an isomeric pair?
View SolutionWhich of the following complexes will show geometrical isomerism ?
View Solution

