Which of the following is incorrect option ?
CuI2+KI→K3[CuI4] complex salt
A
oxidation state of central atom decreases during the formation of complex.
B
Complex is tetrahedral.
C
Hybridisation of the complex is sp3.
D
Hybridisation of the complex is dsp2.
Text Solution
The correct Answer is:D
K3[+CuI4],IC.F.S.E.is very small(since C.A. also has low charge)⇒C.N.=4→sp3
|
Similar Questions
Knowledge Check
Which of the following option is incorrect
AIst ionisation potential : Na<Mg<Si<PBElectron affinity : N<C<O<F<ClCHydrated radius : Ba2+(aq)<Sr2+(aq)<Mg2+(aq)DPolarity order : N−H<Sb−H<As−H<P−HWhich of the following option is incorrect
AIst ionisation potential : Na<Mg<Si<PBElectron affinity : N<C<O<F<ClCHydrated radius : Ba2+(aq)<Sr2+(aq)<Mg2+(aq)DPolarity order : N−H<Sb−H<As−H<P−HWhich of the following options is incorrect?
ApKa1 cis but-2-ene dioic acid gt trans but-2-ene dioic acidBdipole moment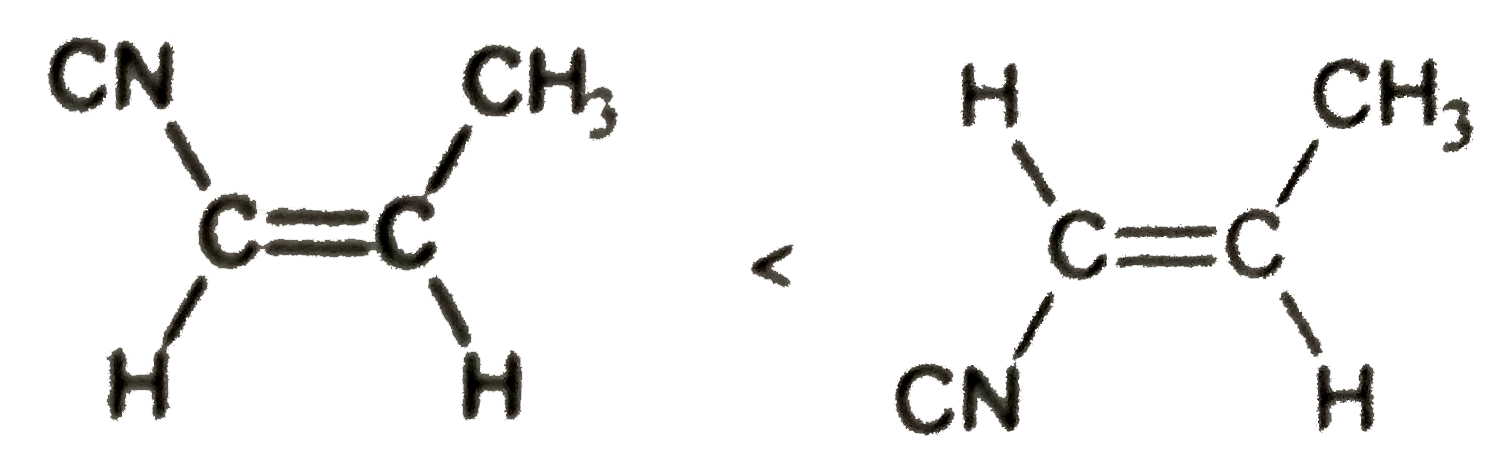 CStability
CStability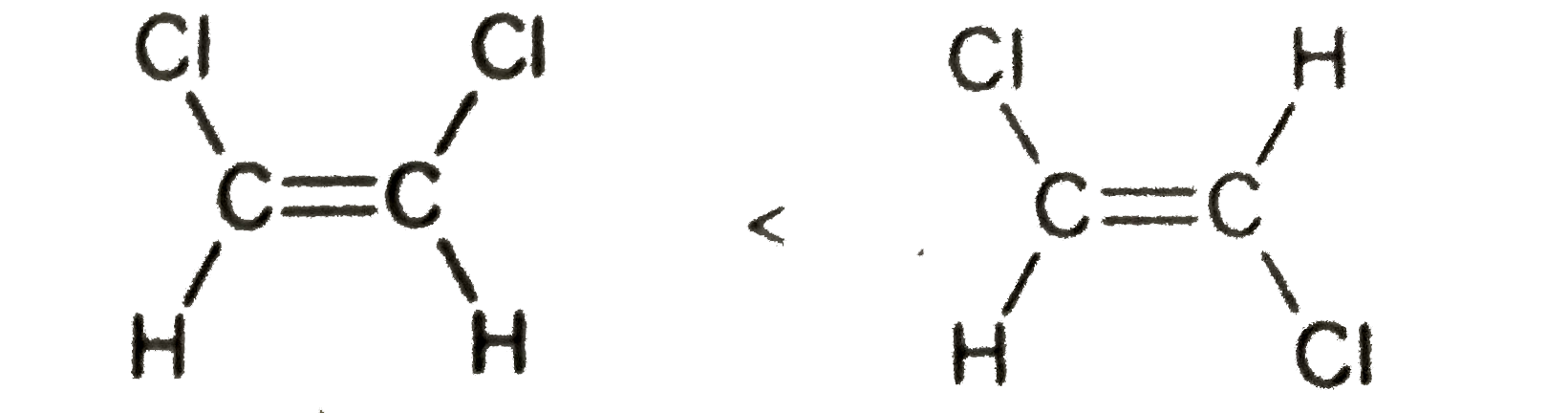 DBasicity
DBasicity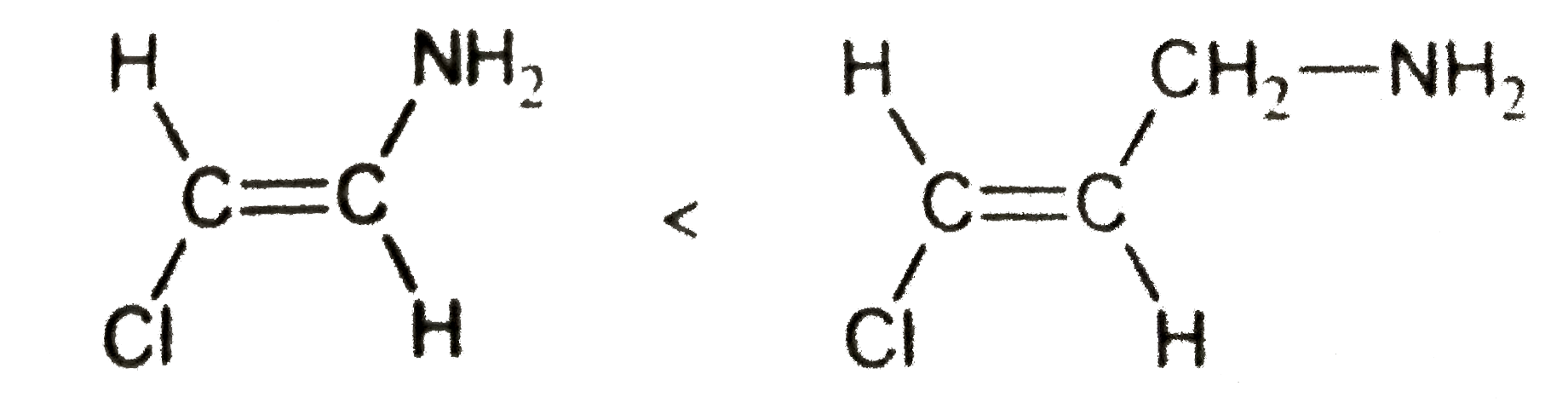
Which of the following option is incorrect?
View SolutionWhich of the following option is incorrect?
View SolutionWhich of the following options are incorrect.
View SolutionWhich of the following is not a double salt but is a complex salt ?
View SolutionWhich options is incorrect for K4[Fe(CN)6]:
View Solution

