All the following complexes show decrease in their weights when placed in a magnetic balance then the group of complexes having tetrahedral geometry is :
(P)Ni(CO)4 (Q)K[AgF4]
(R)Na2[Zn(CN)4] (S)K2[PtCl4]
(T)[RhCl(PPh3)3]
Q, R, T
P, Q, R
P, R, S
None of these
The correct Answer is:D
(P) and (R ) are tetrahedral.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - E. Stability, Colour and Magnetic Properties of Complexes
60 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - F.Isomerism in Coordination Compounds
120 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - C. Werners Theory, EAN Rule
63 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS BOOK - OP TANDONCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS EXERCISE - INTEGER
2 Videos
- ELECTROCHEMISTRYBOOK - OP TANDONCHAPTER - ELECTROCHEMISTRYEXERCISE - Matrix-Matching Type Question
3 Videos
Similar Questions
Among the following ions, which one has the highest paramagnetism ?
View SolutionThe complexes [Pt(NH3)4][PtCl6] and [Pt(NH3)4Cl2][PtCl4] are :
View SolutionEAN of a metal carbonyl M(CO)x is 36. If atomic number of metal M is 26, what is the value of x?
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionThis section contains 3 questions. Each question contains statement given in two columns which have to be matched. Statement (a, b, c and d) in Column-I have to be matched with statement (p, q, r and s) in Column-II. The answers to these questions have to be appropriately bubbled as illustrated in the following examples :
If the correct matches are (a-p, s), (b-q, r), (c-p, q) and (d-s), then correct bubbled 4×4 matrix should be as follows:
Match the column-I with Column-II :
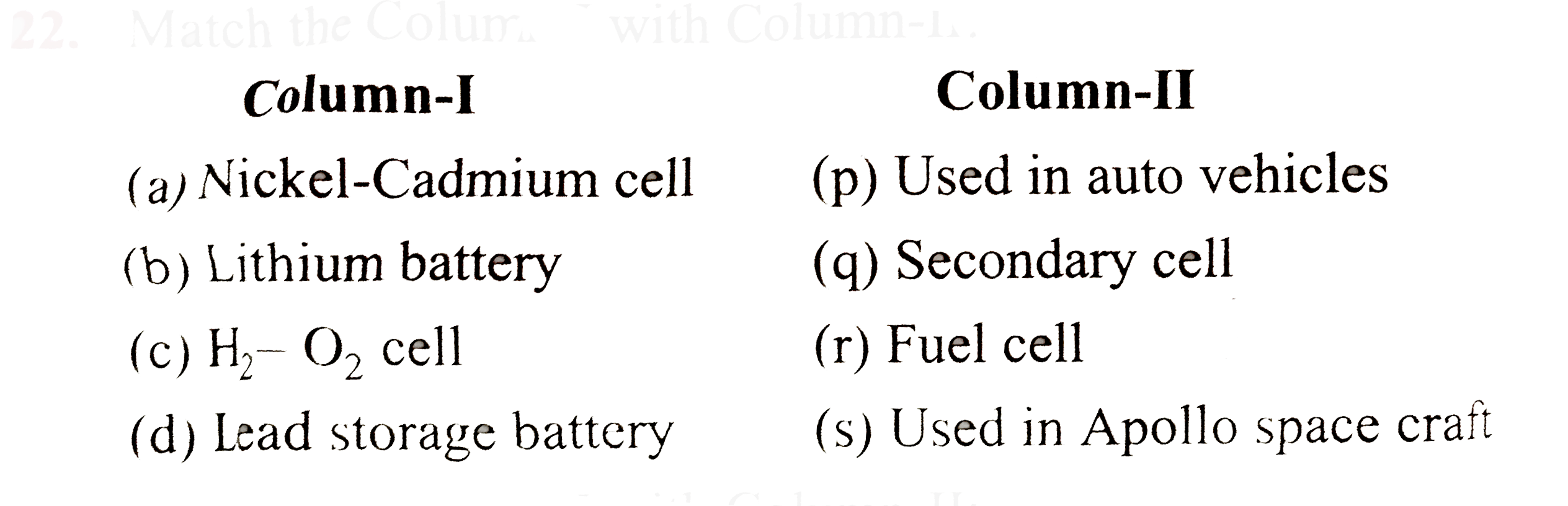
View SolutionThe geometries of Ni(CO)_(4) and Ni(PPh_(3))_(2)CI_(2) are .
View SolutionThe geometries of [Ni(CO)_(4)] and [NiCI_(2)(PPh_(3))-(2)] are
View SolutionAll the following complexes show decrease in their weights when placed in a magnetic balance then the group of complexes having tetrahedral geometry is : Ni(CO)_(4) K[AgF_(4)] Na_(2)[Zn(CN)_(4)] K_(2)[PtCl_(4)] [RhCl(PPh_(3))_(3)]
View Solutionwhich of the following complexes entities are squre planar in shape ?
[Ni(CN)4]−2[MnCl4]−2[Zn(NH3)4]+2 (P) (Q) (R)
[PdCl4]−2[Ni(NH3)4]+2 (S) (T)
View SolutionThe geometry of [Ni(CO)4] and [NiCl2(PPh3)2] are :
View SolutionConsider the following complexes : (P) K_(2)PtCl(6)" "(Q) PtCl_(4).2NH_(3) (R) PtCl_(4).3NH_(3)" "(S) PtCl_(4).5NH_(3)
View SolutionAll the following complexes show a decrease in their weights when placed in a magnetic balance. Then Which of these has square planar geometry?
View SolutionConsider the following complexes : [NiCl_(4)]^(2-), [Ni(CN)_(4)]^(2-), [Ni(CO)_(4)]^(0) What is the similarity in all three complexes ?
View SolutionAll the following complexes show a decrease in their weights when placed in a magnetic balance. Then Which of these has square planar geometry?
View SolutionConsider the following complexes :
[NiCl4]2−,[Ni(CN)4]2−,[Ni(CO)4]0
What is the similarity in all three complexes ?
View Solution

