The complex ion has two optical isomers. Their correct configuration are :
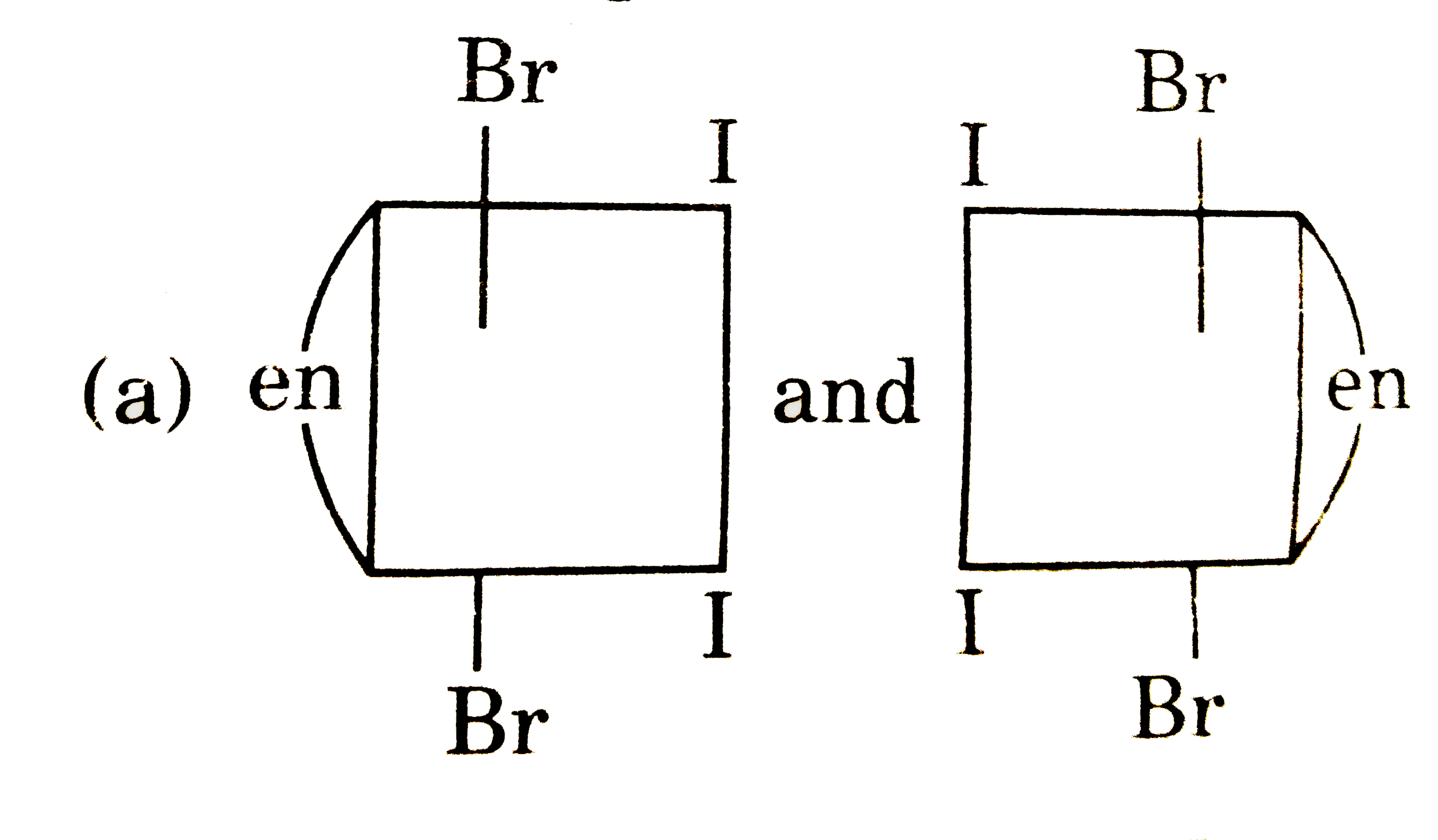
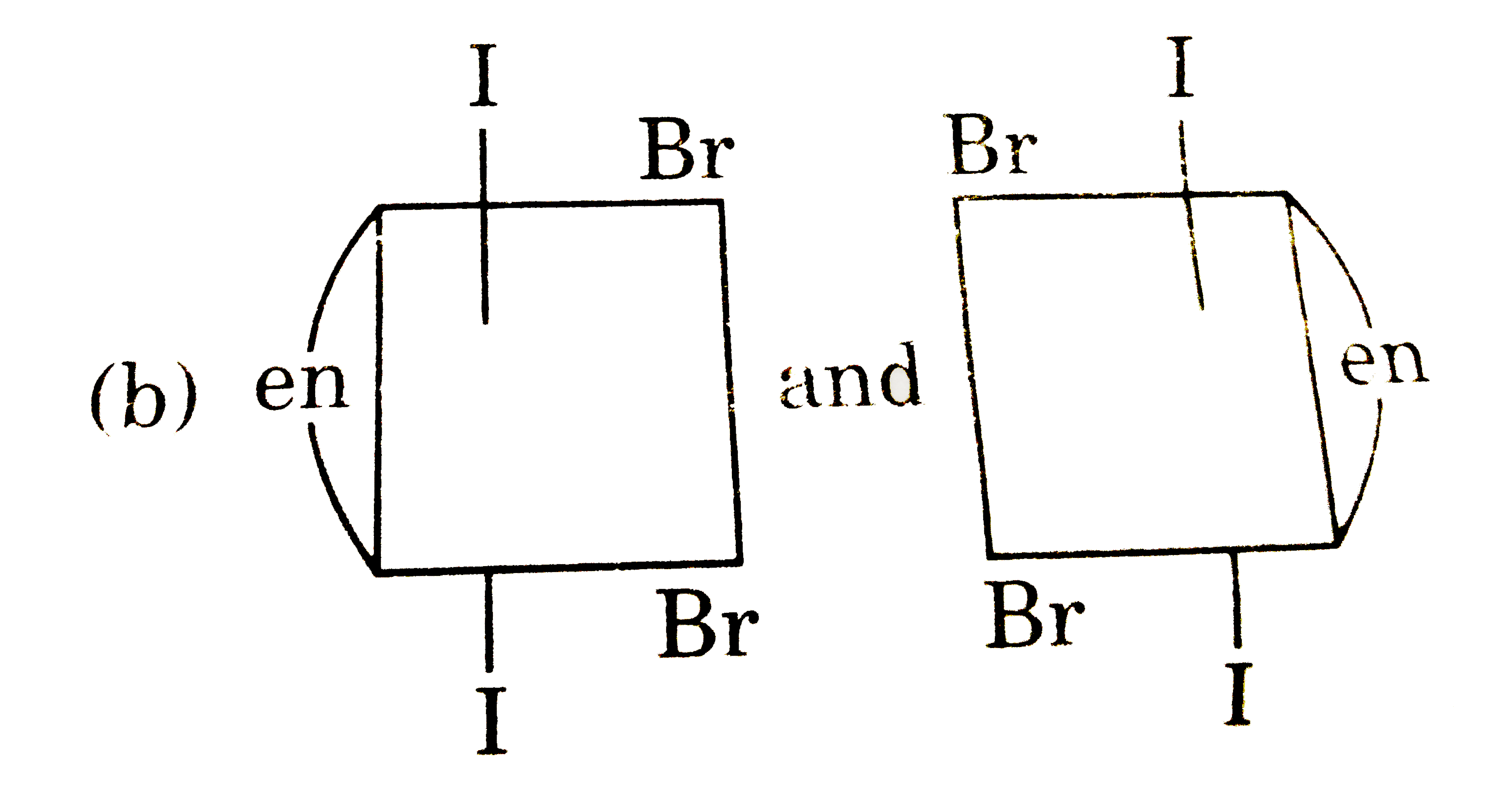
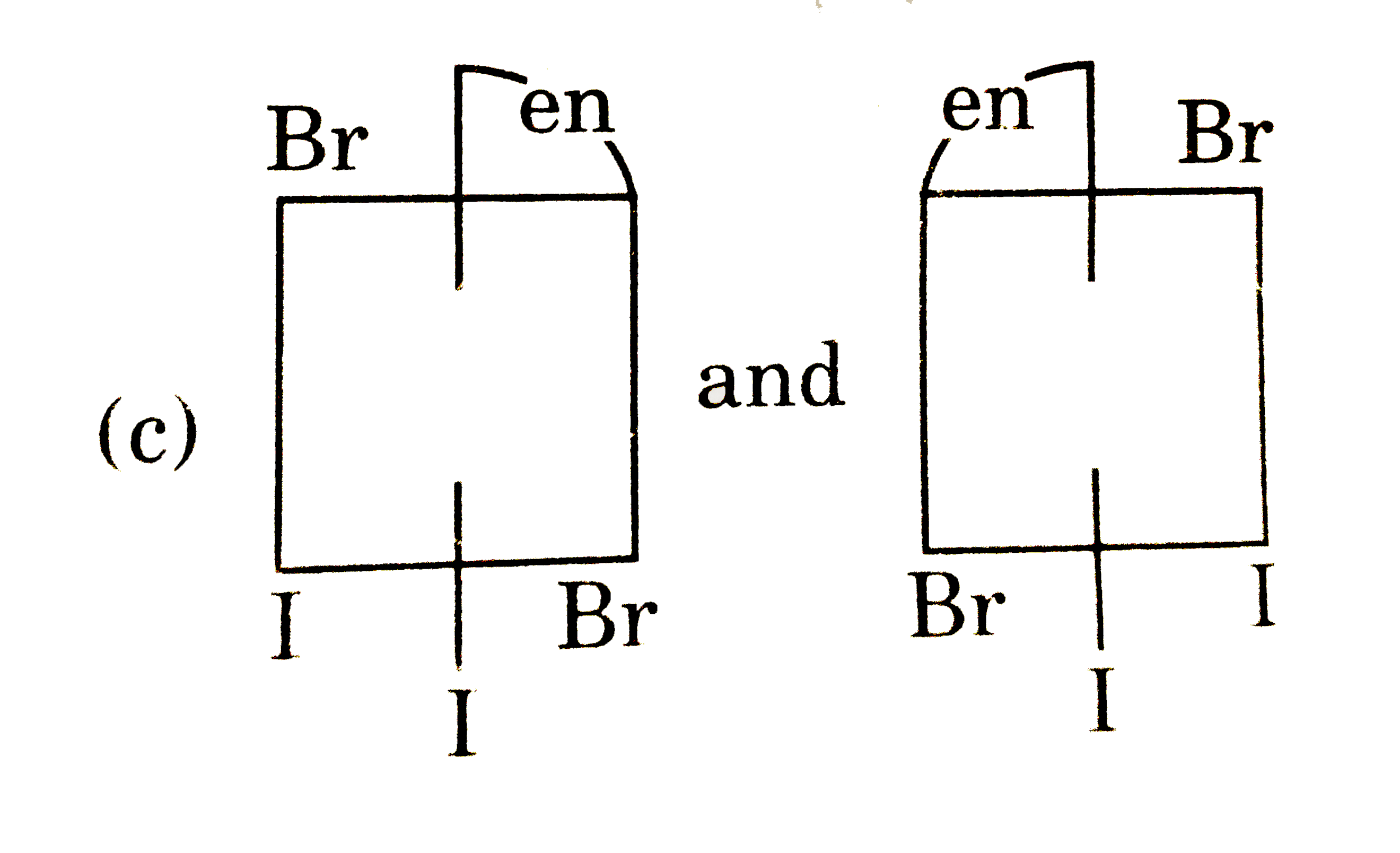
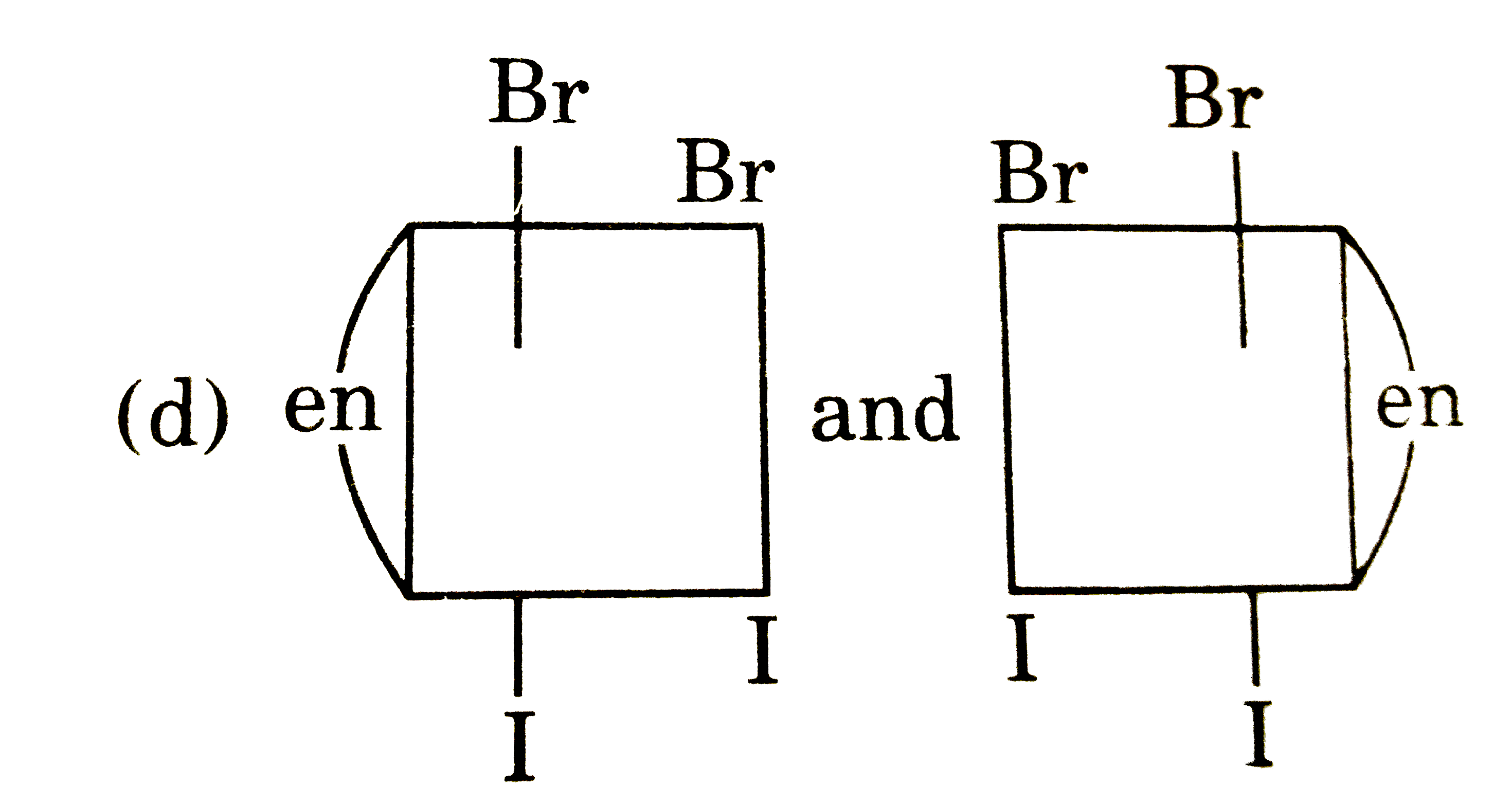
The correct Answer is:D
(d) does not have COS or POS
Similar Questions
Knowledge Check
The complex ion . [M(en)Br2I2]−1, has two optical isomers. Their correct configurations are:
A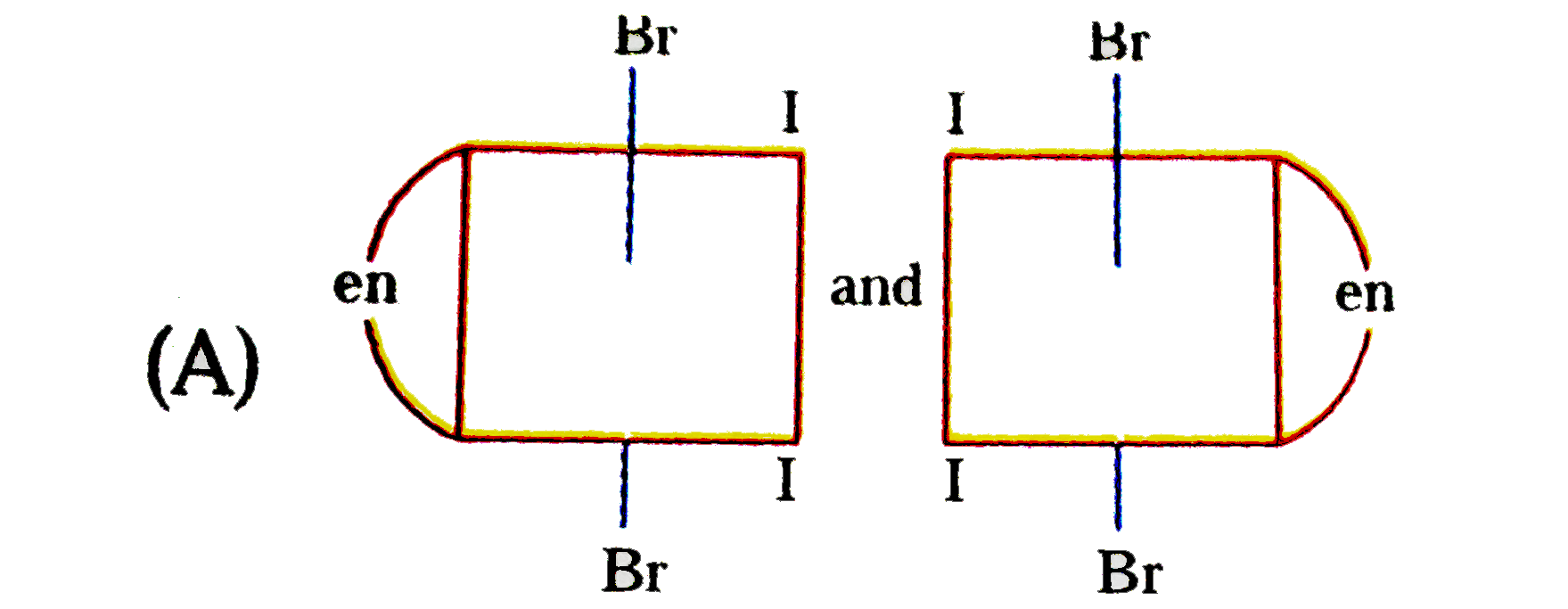 B
B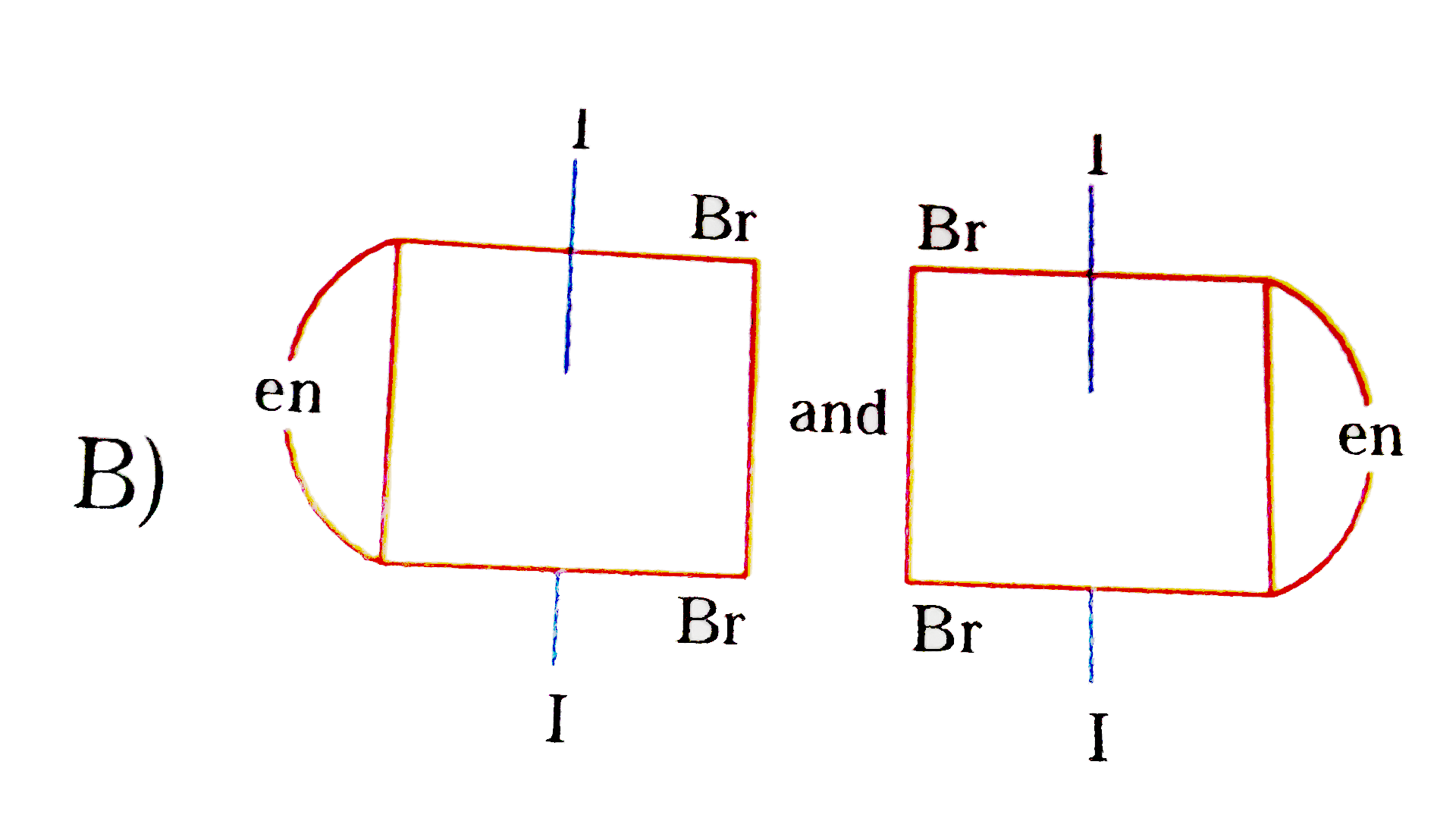 C
C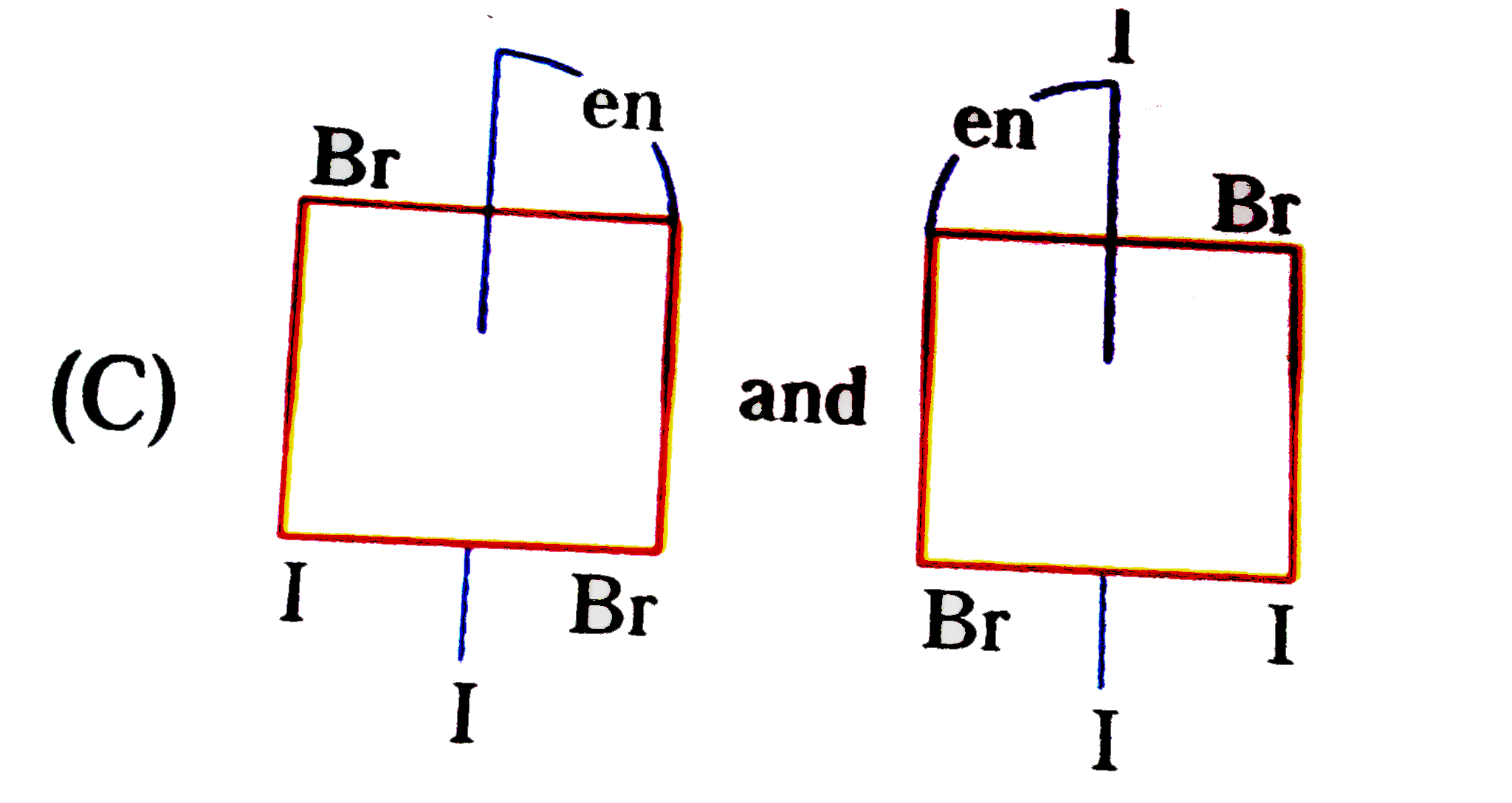 D
D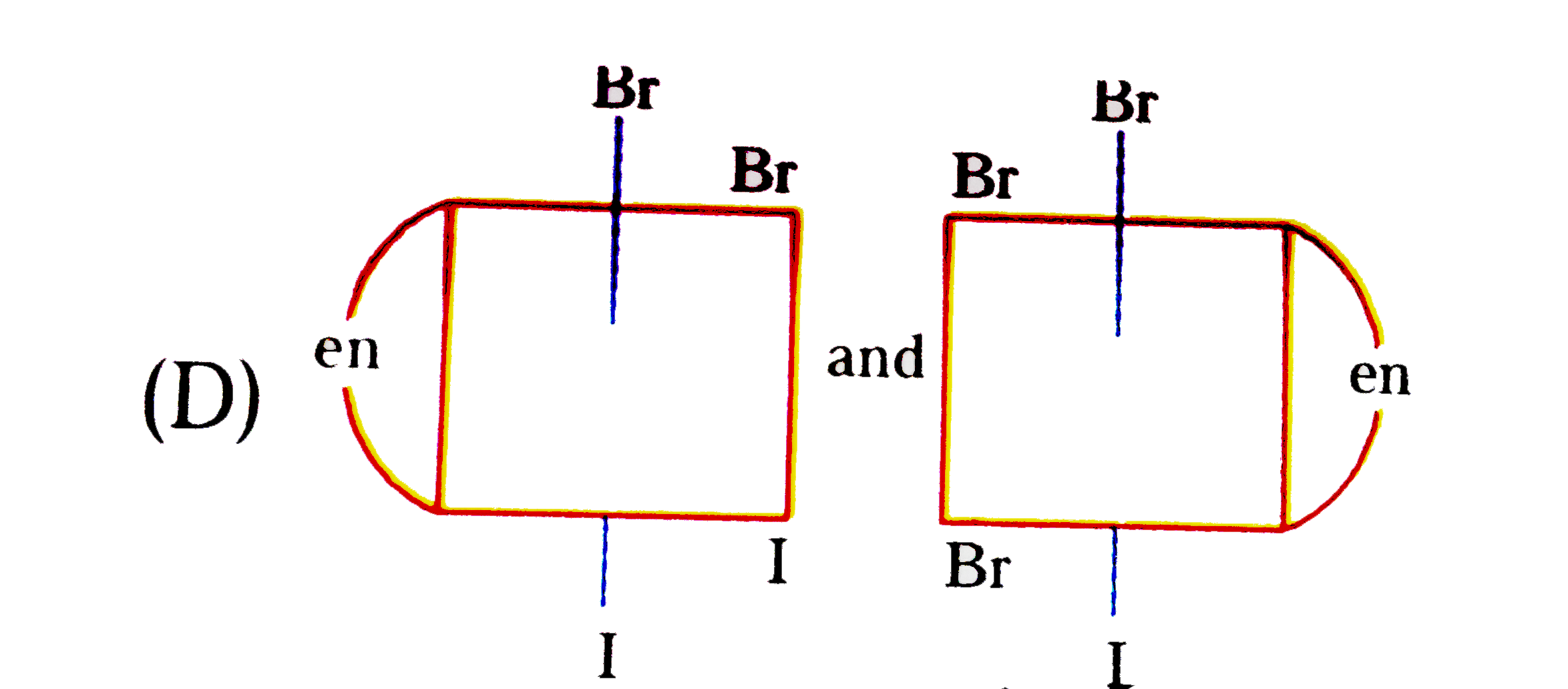
Glucose has optical isomers
A8B12C16DCannot be predictedGlucose has _______ optical isomers.
A4B8C16D10
Glucose has how many optical isomers ?
View SolutionGlucose has how many optical isomers?
View SolutionWhich complex ion has cis and trans isomer ?
View SolutionFour statements for the following reaction given below [CoCl2(NH3)4]+→[CoCl3(NH3)3]+NH3
(P) Only one isomer is produced if the ractant complex ion is a trans isomer
(Q) Three isomers are produced if the reactant complex ion is a cis isomer
(R ) Two isomers are produced if the reactant complex ion is trans isomer
(S) Two isomers are produced if the reactant complex is cis isomer
The correct statements are :
View SolutionCosider the following reaction and statements:
[Co(NH3)4Br]++Br−→[Co(NH3)3Br]+NH3
(I) Two isomers are produced if the reactant complex ion is a cis-isomer.
(II) Two isomers are produced if the reactant complex ion is a tran-isomer.
(III) Only one isomers is produced if the reactant complex ion is a tran-isomer.
(IV) Only one isomers is produced if the reactant complex ion is a cis-isomer.
The correct statements are :
View Solution

