Which of the following isomers of [M(NH3)2Cl2] would react with silver oxalate (Ag2C2O4) ?
A
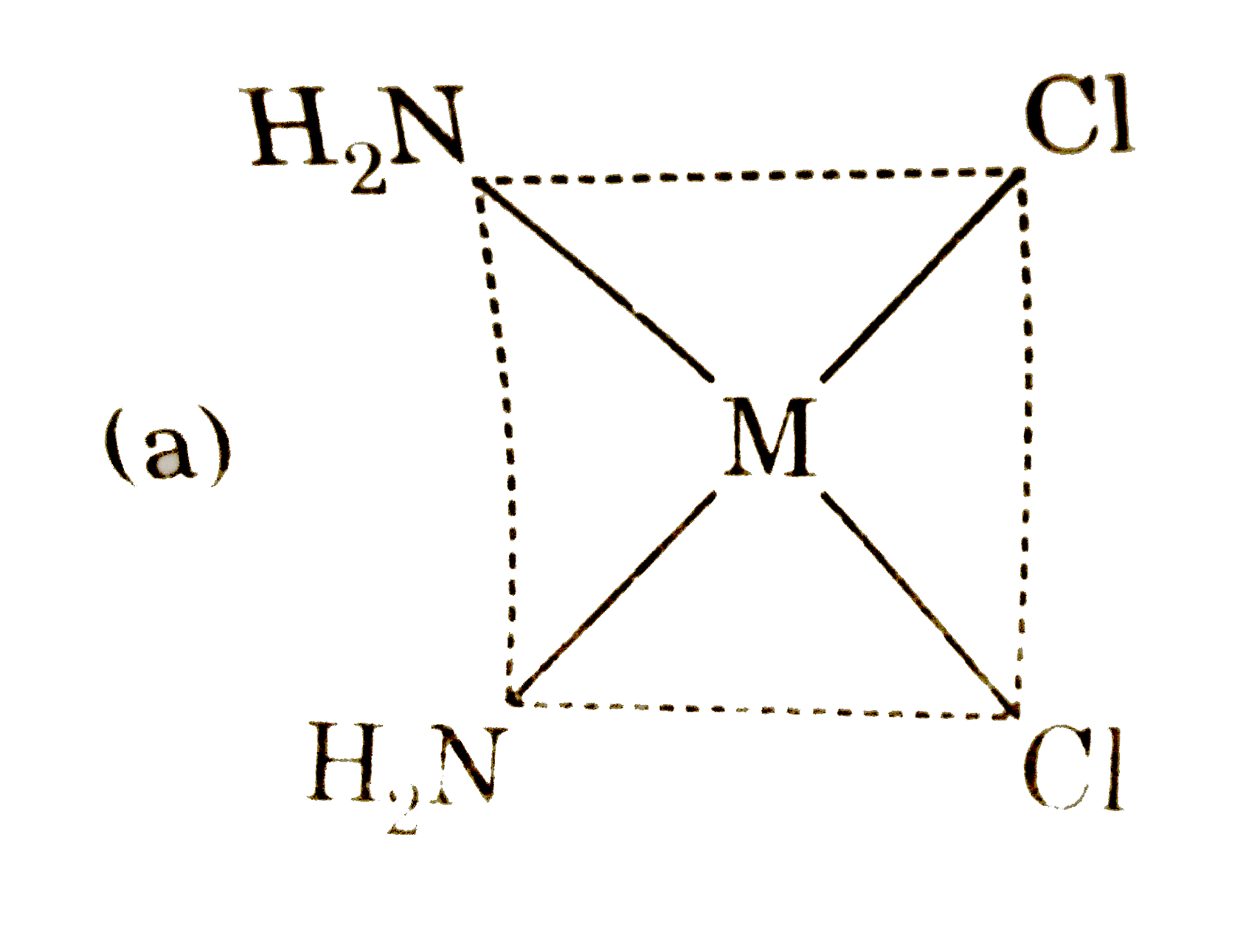
B
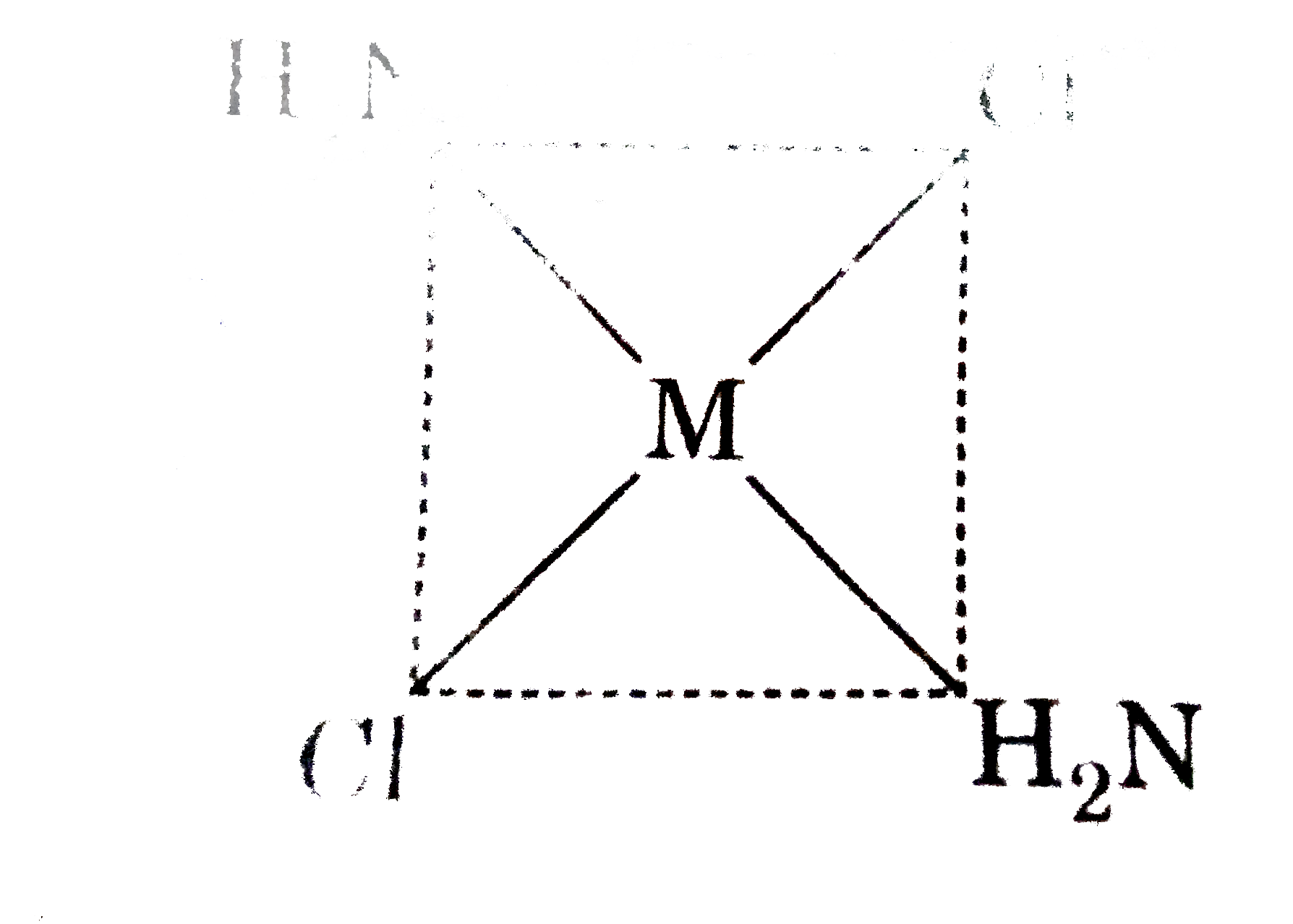
C
Both (a) and (b)
D
None of these
Text Solution
The correct Answer is:A
Only in the cis isomer, 2 monodentate ligands can be displaced by one bidentate ligand.
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:G. Organometallic Compounds, Synergic Bonding
Explore 52 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:REASONING TYPE
Explore 59 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:E. Stability, Colour and Magnetic Properties of Complexes
Explore 60 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Book:OP TANDONChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Exercise:INTEGER
Explore 2 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:Matrix-Matching Type Question
Explore 3 Videos
Similar Questions
Knowledge Check
Which of following isomers of [M(NH3)2Cl2] would react with silver oxalate (Ag2C2O4) :-
A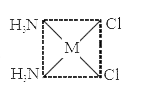 B
B CBothDNone
CBothDNoneWhich of the following isomers of [M(NH3)2Cl2] would react with potassium oxalate (K2C2O4):
A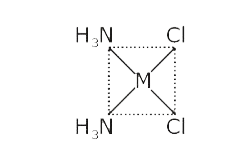 B
B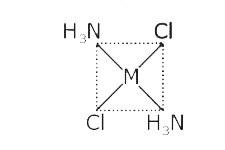 CBothDNone
CBothDNoneThe number of geometrical isomers for [P(NH3)2Cl2] is
A2B1C3D4
Draw the structure of isomes of Pt(NH3)2Cl2
View SolutionDraw the geometrical isomers of complex [Pt(NH3)2Cl2].
View SolutionWhich of the following product is are obtained when Cl2O reacts with NH3?
View SolutionConsider the following isomers.
(i) [Pt(NH3)4Cl2]Br2 (ii)[Pt(NH3)4Br2]Cl2
(iii) [Co(NH3)4Cl2]NO2
Which of the following observations is correct ?
View SolutionConsider the following isomers.
(i) [Pt(NH3)4Cl2]Br2 (ii)[Pt(NH3)4Br2]Cl2
(iii) [Co(NH3)4Cl2]NO2
Which of the following observations is correct ?
View Solution

