Indicate the complex ion which shows geometrical isomerism :
A
[Cr(H2O)4Cl2]+
B
[Pt(NH3)3Cl]+
C
[Co(NH3)6]3+
D
[Co(CN)5(NC)]3−
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - G. Organometallic Compounds, Synergic Bonding
52 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - REASONING TYPE
59 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - E. Stability, Colour and Magnetic Properties of Complexes
60 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS BOOK - OP TANDONCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS EXERCISE - INTEGER
2 Videos
- ELECTROCHEMISTRYBOOK - OP TANDONCHAPTER - ELECTROCHEMISTRYEXERCISE - Matrix-Matching Type Question
3 Videos
Similar Questions
Knowledge Check
Indicate the complex ion which shows geometrical isomerism.
A[Cr(H2O)4)Cl2]+B[Pt(NH3)3Cl]C[Pt(NH3)6]3+D[CO(CN)5(NC)]3−Which show geometrical isomerism?
A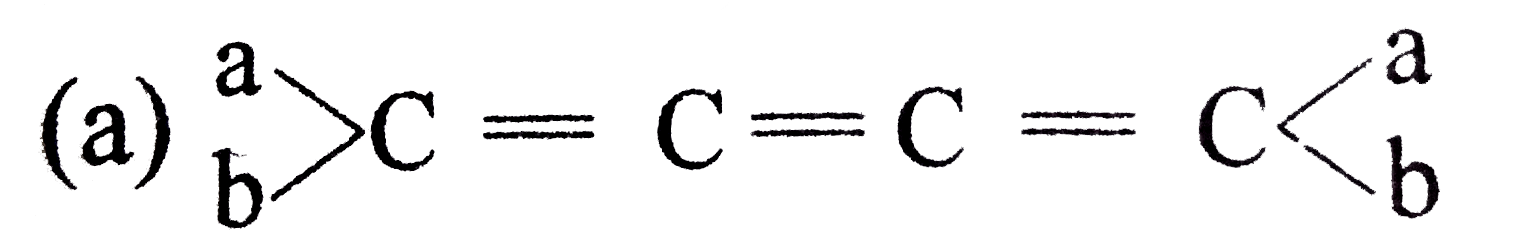 B
B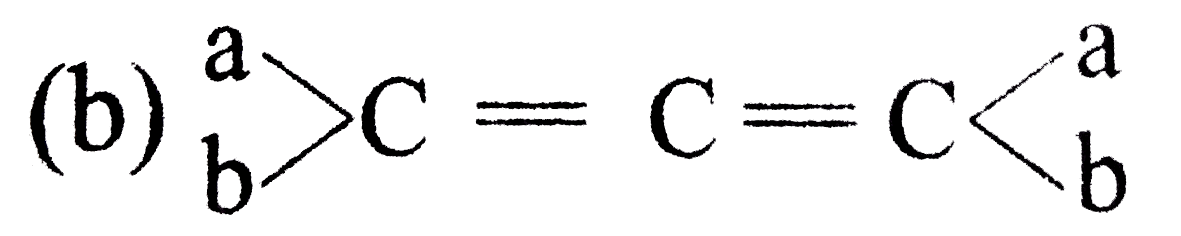 C
C DNone of these
DNone of theseSelect the complex ion which shows geometrical isomerism.
A[Pt(NH3)3Cl]+B[Cr(H2O)4Cl2]+C[Co(NH3)6]3+D[Co(CN)5(NH)]3−