Which of the following factor is responsible for the Π character in metal carbon bond (M-C) in carbonyl complexes ?
Overlap and donation from the lone pair of C into a vacant (hybrid) metal orbital to form a M←C bond,
Overlap and the donation from a filled dxz or dyz orbital on M into a vacant antibonding π∗p orbital on CO to form a M→C bond
Overlap and the donation from the lone pair on vacant (hybrid) metal orbital into C to form a M→C bond
Overlap and the donation from a filled vacant antibonding π∗p orbital on C into dxz or dyz orbital on M to form M←C bond
The correct Answer is:B
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:REASONING TYPE
Explore 59 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:MULTIPLE OBJECTIVE TYPE QUESTION
Explore 129 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:F.Isomerism in Coordination Compounds
Explore 120 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Book:OP TANDONChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Exercise:INTEGER
Explore 2 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:Matrix-Matching Type Question
Explore 3 Videos
Similar Questions
Statement-1: In [Co(en)3]Cl3, the coordination number of Co3+ ion is 3.
Statement-2: 'en' is a bidentate ligand.
View SolutionWhich of the following complexes or molecules has/ have N-N bond length greater than that in free N2 molecule?
View SolutionThe complexes [Pt(NH3)4][PtCl6] and [Pt(NH3)4Cl2][PtCl4] are :
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionThis section contains 3 questions. Each question contains statement given in two columns which have to be matched. Statement (a, b, c and d) in Column-I have to be matched with statement (p, q, r and s) in Column-II. The answers to these questions have to be appropriately bubbled as illustrated in the following examples :
If the correct matches are (a-p, s), (b-q, r), (c-p, q) and (d-s), then correct bubbled 4×4 matrix should be as follows:
Match the column-I with Column-II :
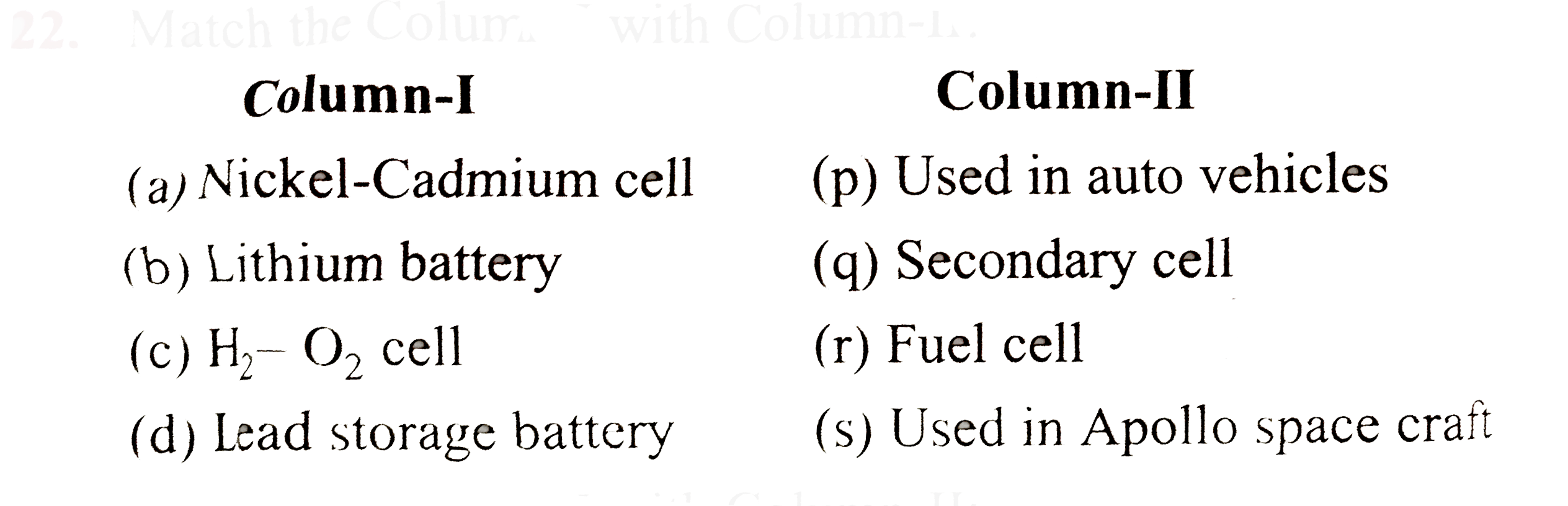
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide.This leads to increase in strength of metal ligand bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide. Simple carbonyls are invariably spin-paired complexes except for vanadium metal. In metal carbonyls, the increase in C-O bond length in CO as compared to carbon monoxide is due to :
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide.This leads to increase in strength of metal ligand bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide. Simple carbonyls are invariably spin-paired complexes except for vanadium metal. Which one of the following metal carbonyls involves the d^2sp^3 hybridisation for the formation of metal-carbon sigma bonds and is paramagnetic ?
View Solution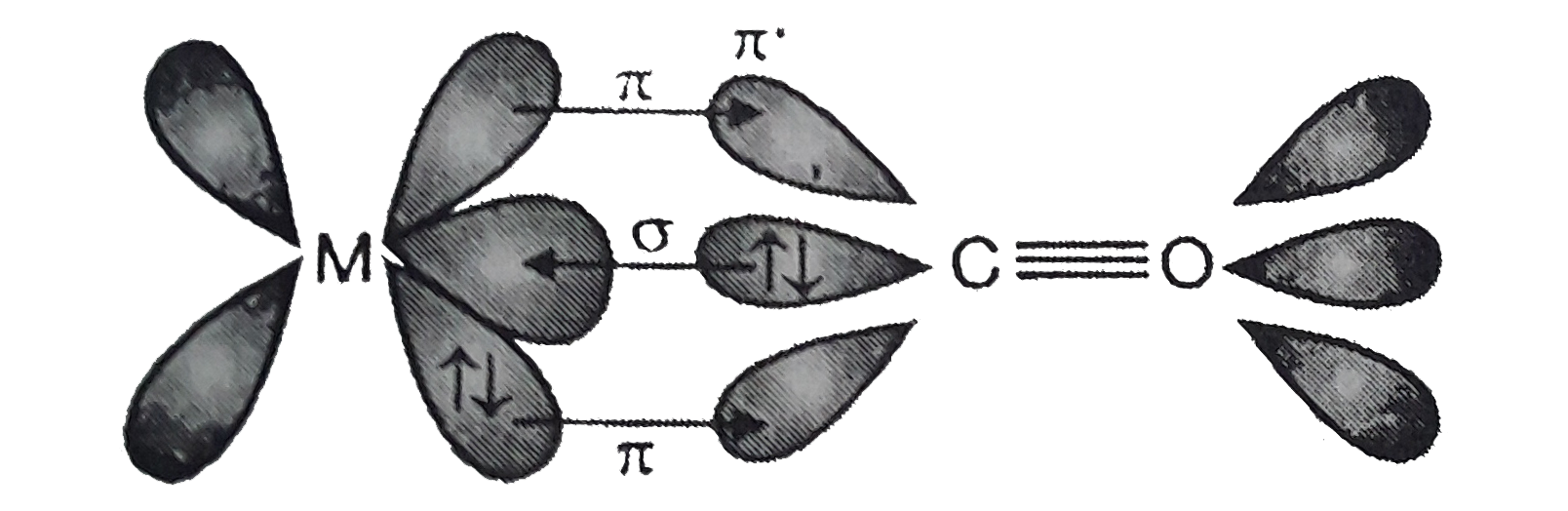
The figure represents the synergic bonding interaction in metal carbonyl complex. On the basis of the explain the following:
(i) Strength of Metal-ligand bond
(ii) Bond order of CO in carbonyl complex as compared to bond order in carbon monoxide.
View SolutionIn metal carbonyl (organometallic ) complexes , the M -C bond is
View SolutionThe metal-carbon bond in metal carbonyls possesses
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide. This leads to increase in strength of metal ligands bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide. Simple carbonyls are invariably spin-paired complexes except for vanadium metal. Which one of the following metal carbonyl involves the d^(2)sp^(3) hybridization for the formation of metal-carbon sigma" "bonds and is paramagnetic ?
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide. This leads to increase in strength of metal ligands bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide.
Simple carbonyls are invariably spin-paired complexes except for vanadium metal.
Which of the following statement is correct for metal carbonyls ?
View SolutionWhich of the following factor is responsible for the π character in metal carbon bond (M−C) in carbonyl complexes ?
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide. This leads to increase in strength of metal ligands bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide.
Simple carbonyls are invariably spin-paired complexes except for vanadium metal.
Which one of the following metal carbonyl involves the d2sp3 hybridization for the formation of metal-carbon σ bonds and is paramagnetic ?
View SolutionIn metal carbonyls, there is synergic bonding interaction between metal and carbon monoxide. This leads to increase in strength of metal ligands bond and decrease in bond order of CO in carbonyl complex as compared to bond order in carbon monoxide. Simple carbonyls are invariably spin-paired complexes except for vanadium metal. Which of the following statement is correct for metal carbonyls ?
View Solution

