For what minimum value of n, polymerisation isomers of [Fe(NH3)3(NO2)3]n would not exist ?
The correct Answer is:6
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:MATCH THE COLUMN TYPE
Explore 11 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Book:OP TANDONChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS Exercise:INTEGER
Explore 2 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:Matrix-Matching Type Question
Explore 3 Videos
Similar Questions
Column IColumn II(a)[Pt(NH3)ClBrI]−(p)Square planar(b)[Pt(NH3)ClBrNO2]−(q)EAN=84(c)[Pt(NH3)Cl(CN)2]−(r)Linkage isomers are 3(t)Linkage isomers are 4
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionThis section contains 3 questions. Each question contains statement given in two columns which have to be matched. Statement (a, b, c and d) in Column-I have to be matched with statement (p, q, r and s) in Column-II. The answers to these questions have to be appropriately bubbled as illustrated in the following examples :
If the correct matches are (a-p, s), (b-q, r), (c-p, q) and (d-s), then correct bubbled 4×4 matrix should be as follows:
Match the column-I with Column-II :
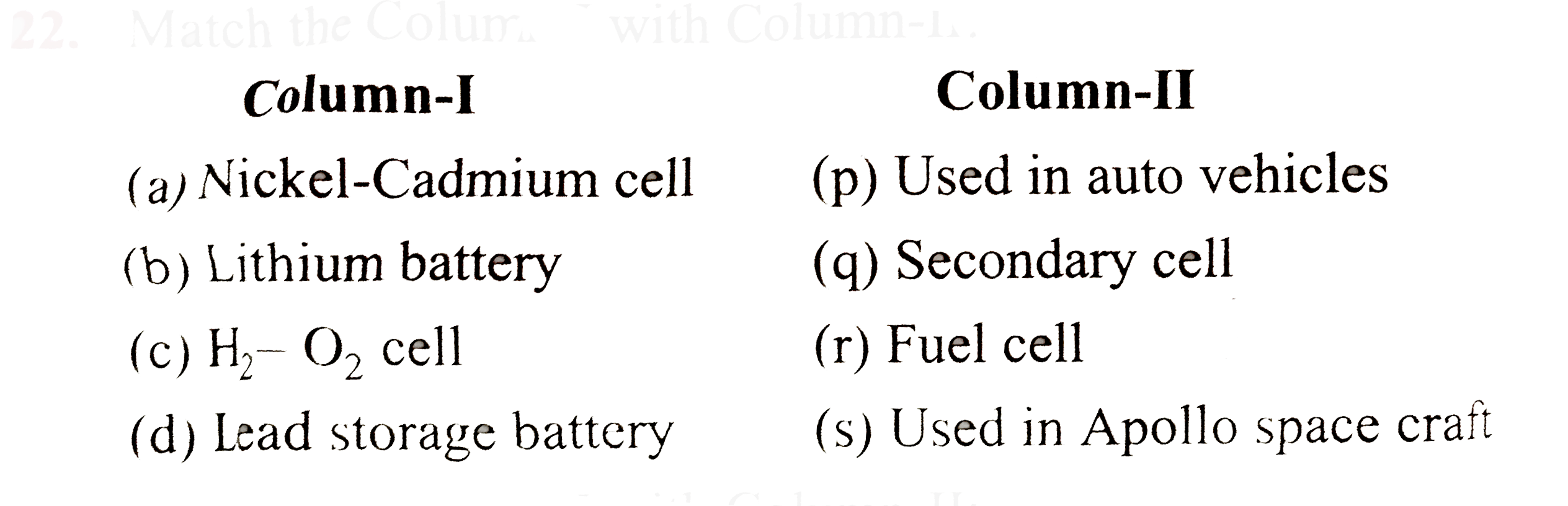
View SolutionThe minimum value of n such that 1+3+32+........+3n≥1000
View SolutionThe ratio of coefficient of HNO_(3), Fe(N)_(3))_(2) and NH_(4)NO_(3) in the following redox reaction Fe + HNO_(3) rarr Fe (NO_(3))_(2) + NH_(4)NO_(3) + H_(2)O are respectively
View SolutionThe number of geometrical isomers of [Co(NH_(3))_(3)(NO_(3))_(3)] is
View SolutionOxidation number of N in NH4NO3 is:
View SolutionThe number of geometrical isomers of [Co(NH_(3))_(3)(NO_(3))_(3)] are :
View SolutionWhich of the following polymerisation isomers of the compound having empirical formula [Cr(NH_(3))_(3)(NO_(2))_(3)]^(+) has the lowest molecular mass ?
View SolutionThe number of geometrical isomers of [Co(NH_(3))_(3)(NO_(3))_(3)] are :
View SolutionComplex compounds which have same molecular formula but have different structural arrangements of ligands around central metal atom or ion are called structural isomers and phenomenon is named as structural isomerism. Which of the following compounds is/are polymerisation isomerof [Fe(NO_(2))_(3)(NH_(3))_(3)] . (P) [Fe(NO_(2))(NH_(3))_(5)][Fe(NO_(2))_(5)(NH_(3))] (Q) [Fe(NO_(2))_(2)(NH_(3))_(4)]_(2)[Fe(NO_(2))_(5)(NH_(3))] (R ) [Fe(NO_(2))(NH_(3))_(5)][Fe(NO_(2))_(4)(NH_(3))_(2)]_(2) (S) [Fe(NO_(2))_(2)(NH_(3))_(4)][Fe(NO_(2))_(4)(NH_(3))_(2)] Choose the correct code :
View SolutionWhich of the following polymerisation isomers of the compound having empirical formula [Cr(NH_(3))_(3)(NO_(2))_(3)]^(+) has the lowest molecular mass ?
View SolutionComplex compounds which have same molecular formula but have different structural arrangements of ligands around central metal atom or ion are called structural isomers and phenomenon is named as structural isomerism.
Which of the following compounds is/are polymerisation isomerof [Fe(NO2)3(NH3)3].
(P)[Fe(NO2)(NH3)5][Fe(NO2)5(NH3)]
(Q)[Fe(NO2)2(NH3)4]2[Fe(NO2)5(NH3)]
(R)[Fe(NO2)(NH3)5][Fe(NO2)4(NH3)2]2
(S)[Fe(NO2)2(NH3)4][Fe(NO2)4(NH3)2]
Choose the correct code :
View SolutionFor what minimum value of n, polymerisation isomers of [Fe(NH3)3(NO2)3]n would not exist ?
View SolutionThe number of geometrical isomers of [Co(NH3)3(NO2)3] are
View Solution

