Study the following experiment and answer the question at the end of it The following reactions were studied at 25∘ C in benzene solution containing 0.10 M pyridine
CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCI
A B C
The following sets of data were observed:
SetInitial concentrationTime differentFinal concentration[C][A][B]0I0.10M0.05M25min0.0033MII0.10M0.10M15min0.0039MIII0.20M0.10M7.5min0.0077M
Rates d[C]dt in sets I, II and III are respectively (in M −1min)
IIIIII1.30×10−42.6×10−41.02×10−3
IIIIII0.0330.00390.0077
IIIIII0.02×10−40.04×10−40.017
IIIIIINone of these
The correct Answer is:A
Topper's Solved these Questions
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Comprehension type 16
Explore 1 Video
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Comprehension type 17
Explore 1 Video
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Comprehension type 13
Explore 1 Video
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:Subjective Type
Explore 120 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:SBJECTIVE TYPE
Explore 98 Videos
Similar Questions
The following data were observed for the following reaction at 25∘C,CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCl
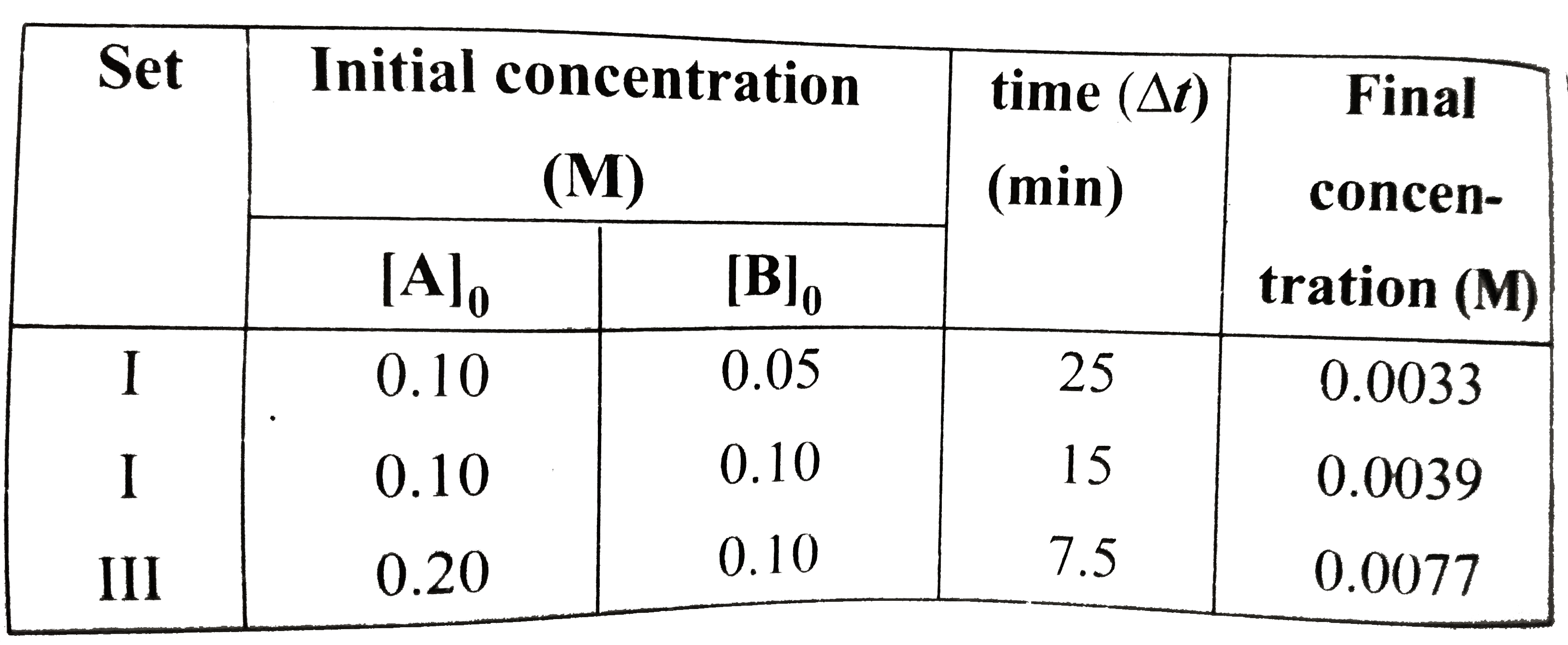
Rate constant of the above experiment is (in L2M−2−1min) :
View SolutionThe following data were observed for the following reaction at 25∘C,CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCl
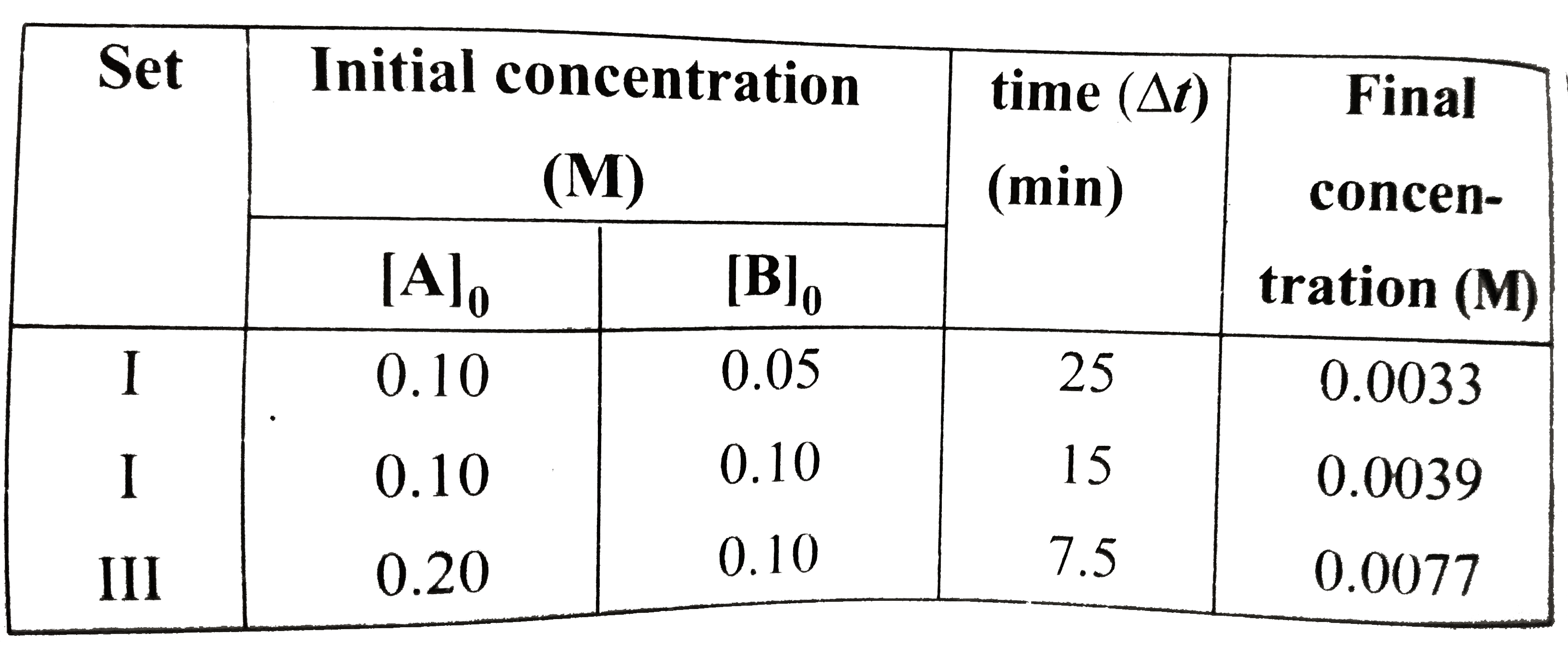
Rates d[C]dt in sets I, II, and III are, respectively,
(in M−1min):
View Solution
Knowledge Check
Study the following experiment and answer the question at the end of it The following reactions were studied at 25∘ C in benzene solution containing 0.10 M pyridine
CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCI
A B C
The following sets of data were observed:
SetInitial concentrationTime differentFinal concentration[C][A][B]0I0.10M0.05M25min0.0033MII0.10M0.10M15min0.0039MIII0.20M0.10M7.5min0.0077M
Rate law of the above experiment is:Ar=k[A][B]Br=k[A]2[B]Cr=k[A][B]2Dr=k[A]2[B]0Study the following experiment and answer the question at the end of it The following reactions were studied at 25∘ C in benzene solution containing 0.10 M pyridine
CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCI
A B C
The following sets of data were observed:
SetInitial concentrationTime differentFinal concentration[C][A][B]0I0.10M0.05M25min0.0033MII0.10M0.10M15min0.0039MIII0.20M0.10M7.5min0.0077M
Rate constant of the above experiment is:A1.3×10−1B2.6×10−2C2.6×10−1D1.3×10−2The following data were observed for the following reaction at 25∘C,CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCl

Rate constant of the above experiment is (in L2M−2−1min) :A2.6×10−2B2.6×10−1C2.6×10−4D1.3×10−2
What are the pH values of the following solutions at 25∘C ?
(i) 0.001 M of HCl
(ii) 0.05 M of H2SO4
(iii) 0.01 M of NaOH
View SolutionIn a solution containing 0.02MCH3COOH and 0.01MC6H5COOH. If KCH3COOH and KC6H5COOH are 1.8×10−5and6.4×10−5 respectively, then calculate the pH of solution.
View SolutionThe following data were observed for the following reaction at 25∘C,CH3OH+(C6H5)3CCl→(C6H5)3C.OCH3+HCl

Rates d[C]dt in sets I, II, and III are, respectively,
(in M−1min):
View SolutionWhat is [H+] in mol/L of a solution that is 0.20M in CH3COONa and 0.1M in CH3COOH? Ka for CH3COOH is 1.8×10−5?
View SolutionWhat is [H+] is mol/ L of a solution that is 0.20 M in CH3COONa and 0.10 M in CH3COOH ? Ka for CH3COOH=1.8×10−5
View Solution

