The electric potential between a proton and as electron is given by V=V0ln(r)r0 , wherer0 is a constant . Assuming Bohr's model to be applicable , write variation of rn with n,n being the principal quantum number ?
rn∝n
rn∝1/n
rn∝n2
rn∝1/n2
The correct Answer is:A
V=V0 In (r/r0)
P.E. U=−eV=−eV=−eV0 In (r/r0)
F=−dUdr=eV0(r/r0).1r0=eV0r
mv2r=eV0r⇒v=√eV0m
mvr=nh2π
r=nh2πmv=nh2πm√eV0m=nh2π√meV0
r=rn∝n
Topper's Solved these Questions
Similar Questions
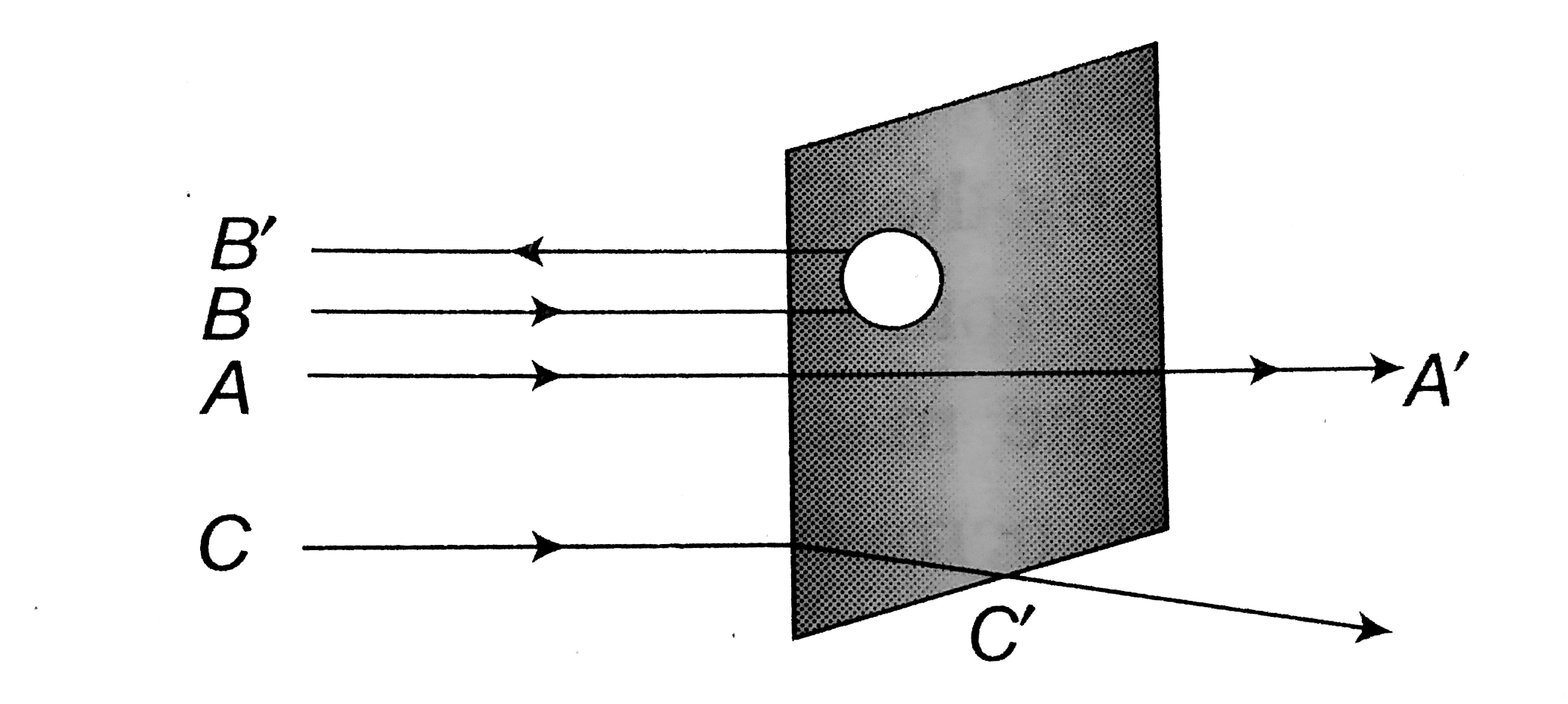
A beam of fast moving alpha particles were directed towards a thin film of gold. The parts A',B' and C' of the transmitted and refected beams correcponding ro the incident parts A,B and C of the beam, are shown in the adjoining diagram. The number of alpha particles in
View SolutionThe peak voltage in a 220VAC source is
View SolutionThe capacitance of a capacitor depends on
(A) Charge on the plates
(B) the size of the plates
(C) the shape of the plates
(D) the separation between the plates
View SolutionThe elecrric potential between a proton and as electron is given by V=V0ln(rr0) , wherer0 is a constant . Assuming Bohr's model to be applicable , write variation of rn with n, n being the principal quantum number ?
View SolutionThe potential of an atom is given by V=V_(0)log_(e)(r//r_(0)) where r_(0) is a constant and r is the radius of the orbit Assumming Bohr's model to be applicable, which variation of r_(n) with n is possible (n being proncipal quantum number)?
View Solutionबोहर परमाणु में, इलेक्ट्रॉन तथा प्रोटॉन के बिच वैधुत विभवान्तर V=V0loge(r)r0 द्वारा दिया गया है, जहाँ r0 नियतांक है। मुख्य क्वाण्टम संख्या n के साथ r0 नियतांक है। मुख्य क्वाण्टम संख्या n के साथ rn का विचरण होगा :
View SolutionThe electric potential between a proton and an electron is given by V=V0In(rr0) where r0 is a constant. Assume that Bohr atom model is applicable to potential, then variation of radius of nth orbit rn with the principal quantum number n is
View SolutionThe electric potential between a proton and an electron is given by V= V_(0) In((r)/(r_(0))) where r_(0) is a constant. Assume that Bohr atom model is applicable to potential, then variation of radius of n^(th) orbit r_(n) with the principal quantum number n is
View SolutionThe electric potential between a proton and an electron is given by V=V0In(rr0) where r0 is a constant. Assume that Bohr atom model is applicable to potential, then variation of radius of nth orbit rn with the principal quantum number n is
View SolutionThe electric potential between a proton and an electron is given by V=V0Inrr0 where r0 is a constant. Asuming Bohr'a model to be applicable, write variation of, rn with n, n being the principal quantum number: --
View SolutionThe electric potential between a proton and an electron is given by V=V0 Inrr0 where r0 is a constant . Assuming Bohr's model to be applicable, write a variation of rn with n, n being the principal quantum number.
View SolutionThe potential of an atom is given by V=V0loge(r/r0) where r0 is a constant and r is the radius of the orbit Assumming Bohr's model to be applicable, which variation of rn with n is possible (n being proncipal quantum number)?
View SolutionThe electric potential between a proton and an electron is given by V=V_(0) In (r/r_(0)), where r_(0) is a constant. Assuming the Bohrmodel to be applicable, write variation ofr with n, n being the principle quantum number?
View SolutionThe electric potential between a proton and as electron is given by V= V_(0) ln (r )/(r_(0)) , where r_(0) is a constant . Assuming Bohr's model to be applicable , write variation of r_(n) with n,n being the principal quantum number ?
View Solutionrn/r0 का अनुपात है (जहाँ rn = नाभिक की त्रिज्या तथा = r0 = परमाणु की त्रिज्या है।)
View Solutionrn/r0 का अनुपात है (जहाँ rn = नाभिक को त्रिज्या तथा r0 = परमाणु की त्रिज्या है।)
View Solution

