In a D-galactopyranose the vicinal hydroxyl groups are cis to each other-
 .
.

CH3−CH∣OH−CH3
CH3COOH
The correct Answer is:A
Step by step video, text & image solution for In a D-galactopyranose the vicinal hydroxyl groups are cis to each other- . by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
An amino acid with a hydroxyl group is
AalanineBtyrosineCvalineDphenyl alanineThe number of hydroxyl groups in glucose is
ASixBFourCFiveDThreeA reaction characteristic of vicinal diols is their oxidative cleavage on treatment with periodic acid (HIO4). The carbon-carbon bond of the vicinal diol uinit is broken and two carbonyl groups result. Periodic acid is reduced to iodic acid (HIO3)
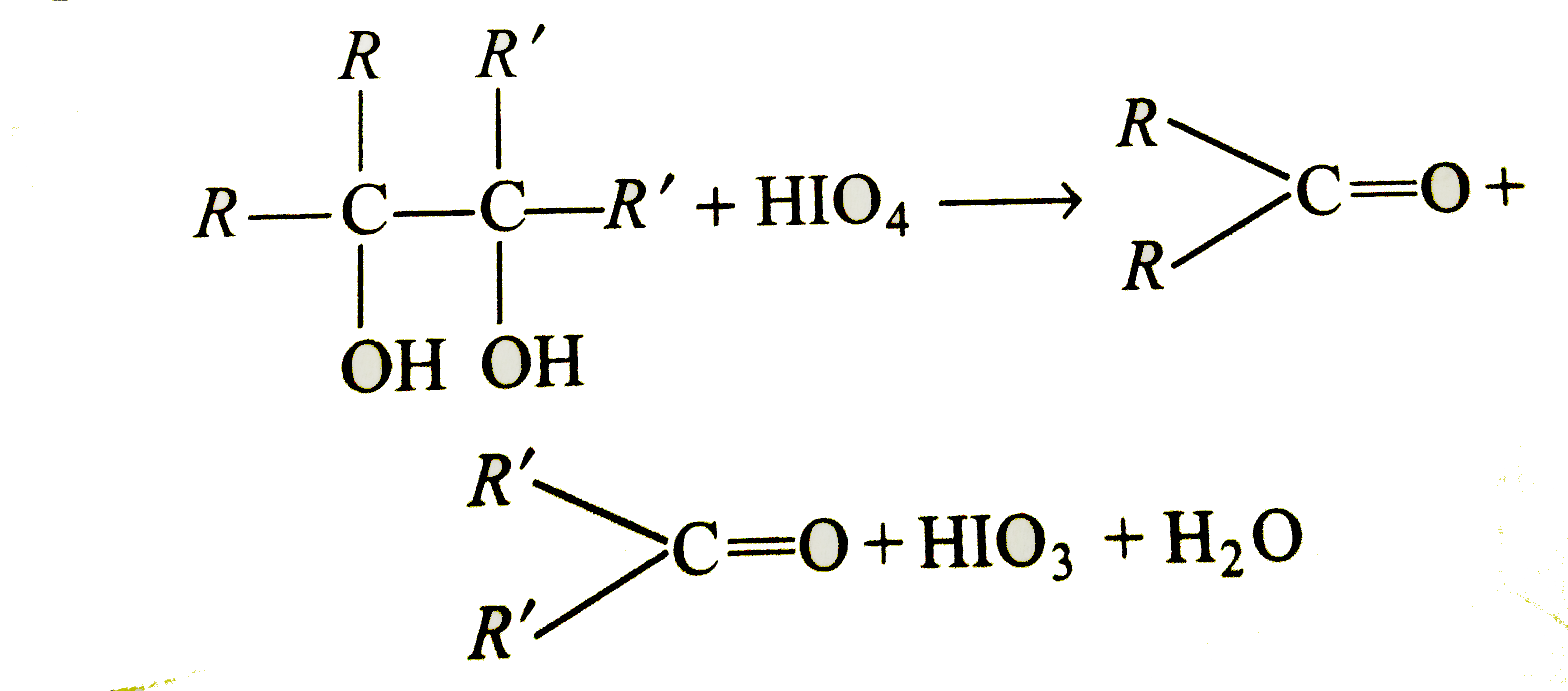
Cyclic diols react to give dicarbonyl compounds. The reactions are faster when the hydroxyl gorups are cid than when they are trans but both stereoisomers are oxidised by periodic acid. Periodic acid cleavage of vicinal diols is often used for analytic purposes as an aid in structure determination. By identifying the carbonyl compounds produced, The constitution of the diol may be deduced.
Which of the following products is/are obtained when glycerol is treated with HIO4 ?AHCHOBHCOOHCHIO3DAll of these
A reaction characteristic of vicinal diols is their oxidative cleavage on treatment with periodic acid (HIO4). The carbon-carbon bond of the vicinal diol uinit is broken and two carbonyl groups result. Periodic acid is reduced to iodic acid (HIO3)
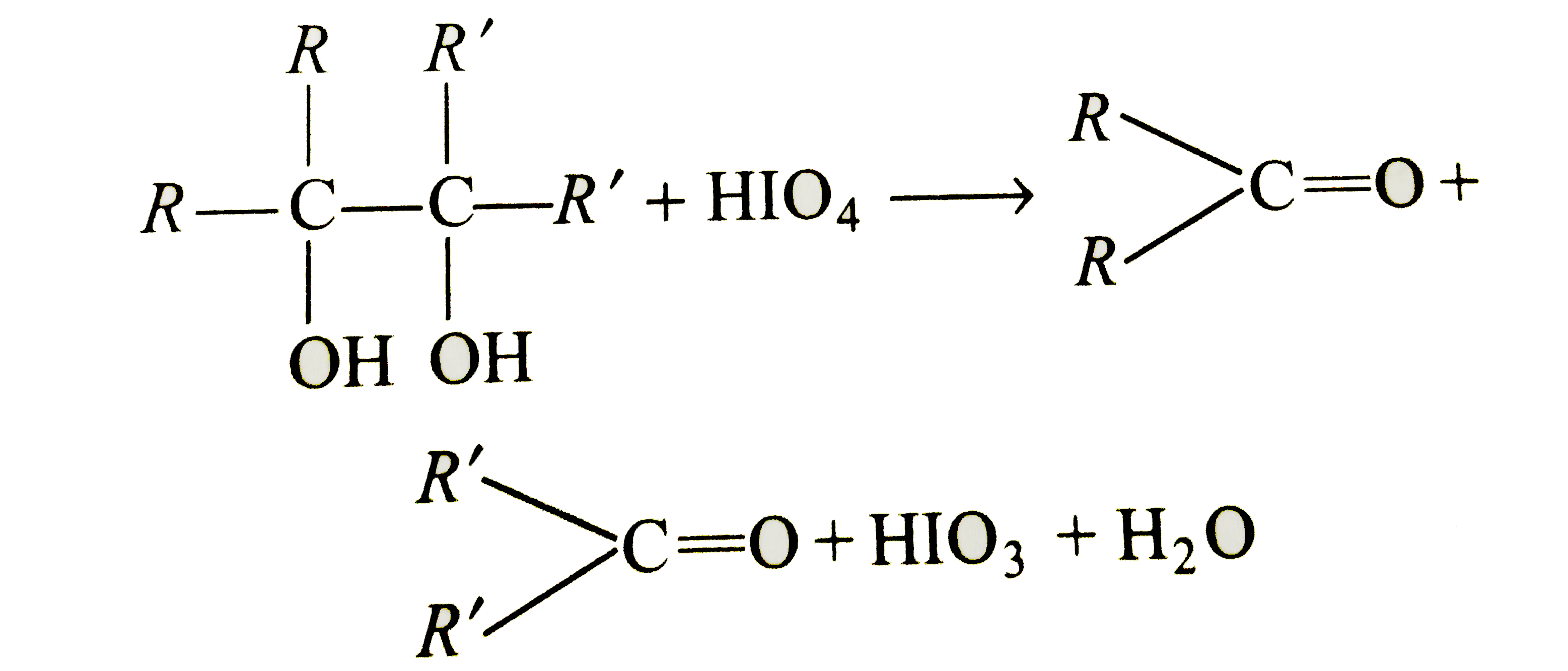
Cyclic diols react to give dicarbonyl compounds. The reactions are faster when the hydroxyl gorups are cid than when they are trans but both stereoisomers are oxidised by periodic acid. Periodic acid cleavage of vicinal diols is often used for analytic purposes as an aid in structure determination. By identifying the carbonyl compounds produced, The constitution of the diol may be deduced.
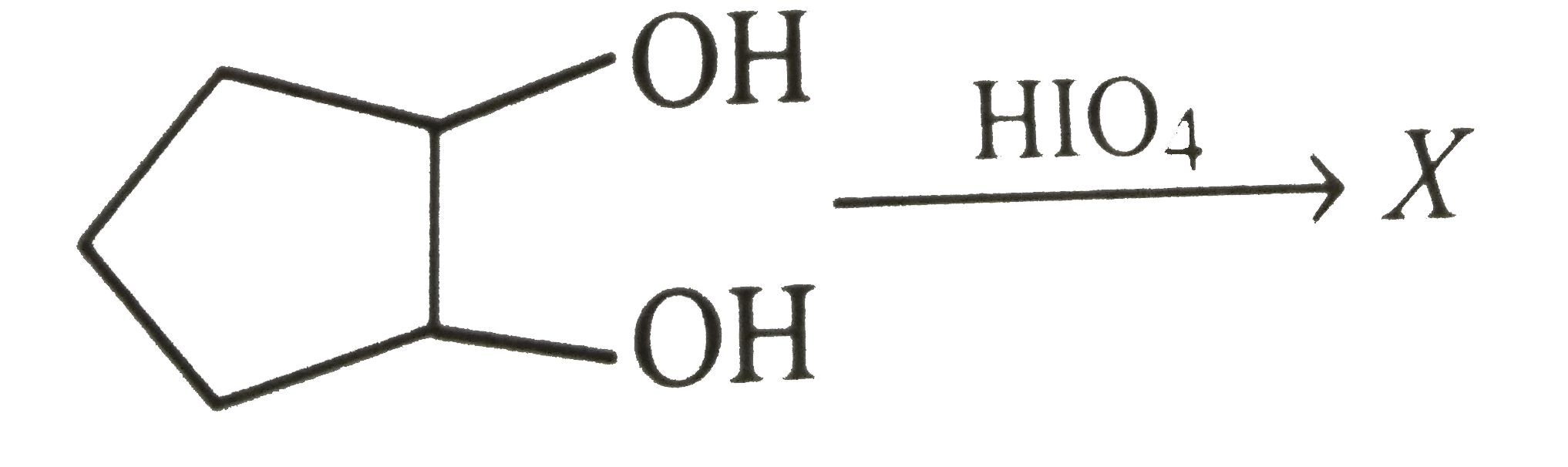
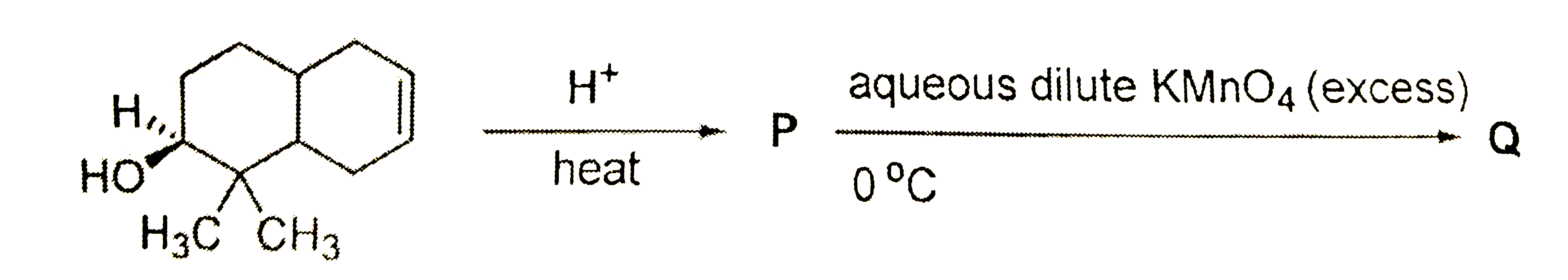
The compound 'X' in above reaction is :
View SolutionA reaction characteristic of vicinal diols is their oxidative cleavage on treatment with periodic acid (HIO4). The carbon-carbon bond of the vicinal diol uinit is broken and two carbonyl groups result. Periodic acid is reduced to iodic acid (HIO3)
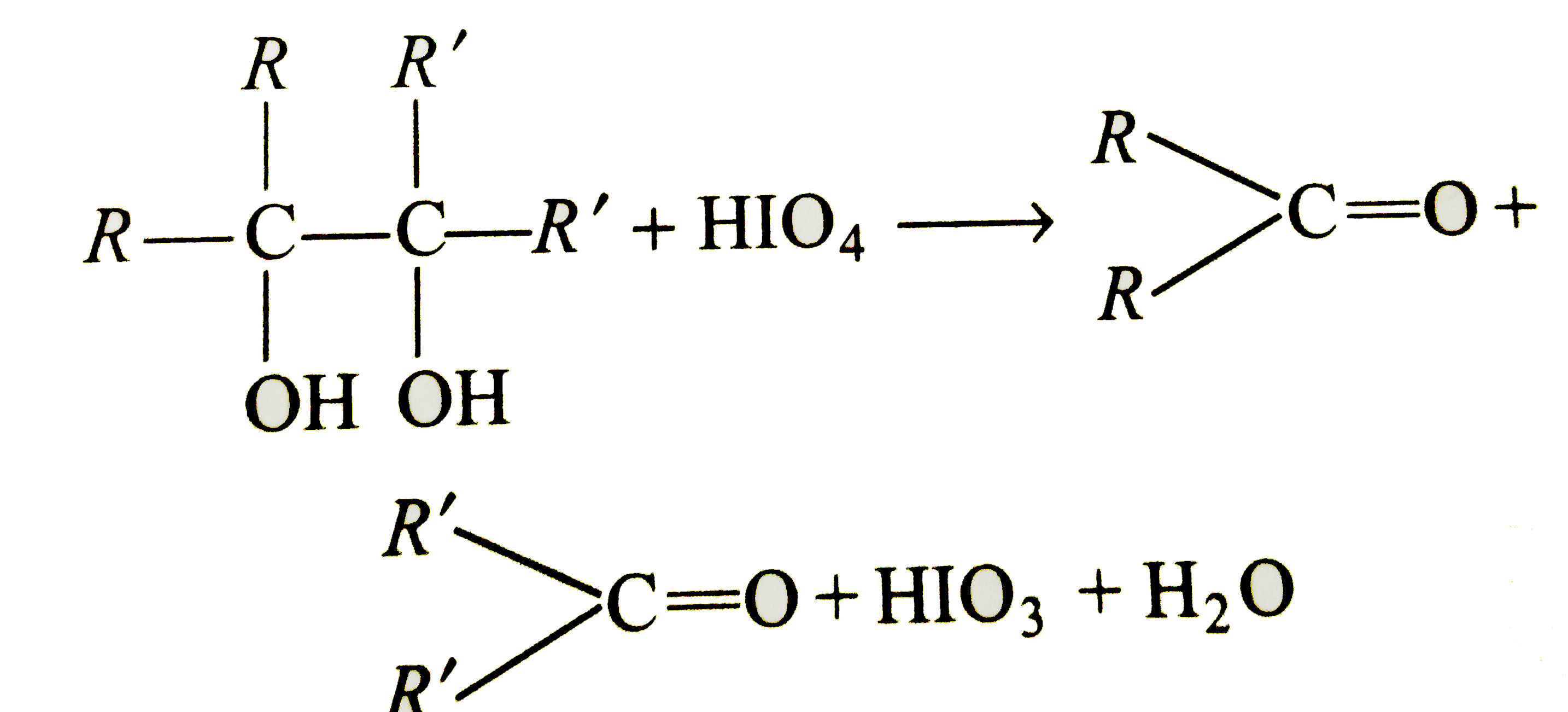
Cyclic diols react to give dicarbonyl compounds. The reactions are faster when the hydroxyl gorups are cid than when they are trans but both stereoisomers are oxidised by periodic acid. Periodic acid cleavage of vicinal diols is often used for analytic purposes as an aid in structure determination. By identifying the carbonyl compounds produced, The constitution of the diol may be deduced.
Which of the following diols will be oxidised by HIO4 ?
View SolutionA reaction characteristic of vicinal diols is their oxidative cleavage on treatment with periodic acid (HIO4). The carbon-carbon bond of the vicinal diol uinit is broken and two carbonyl groups result. Periodic acid is reduced to iodic acid (HIO3)
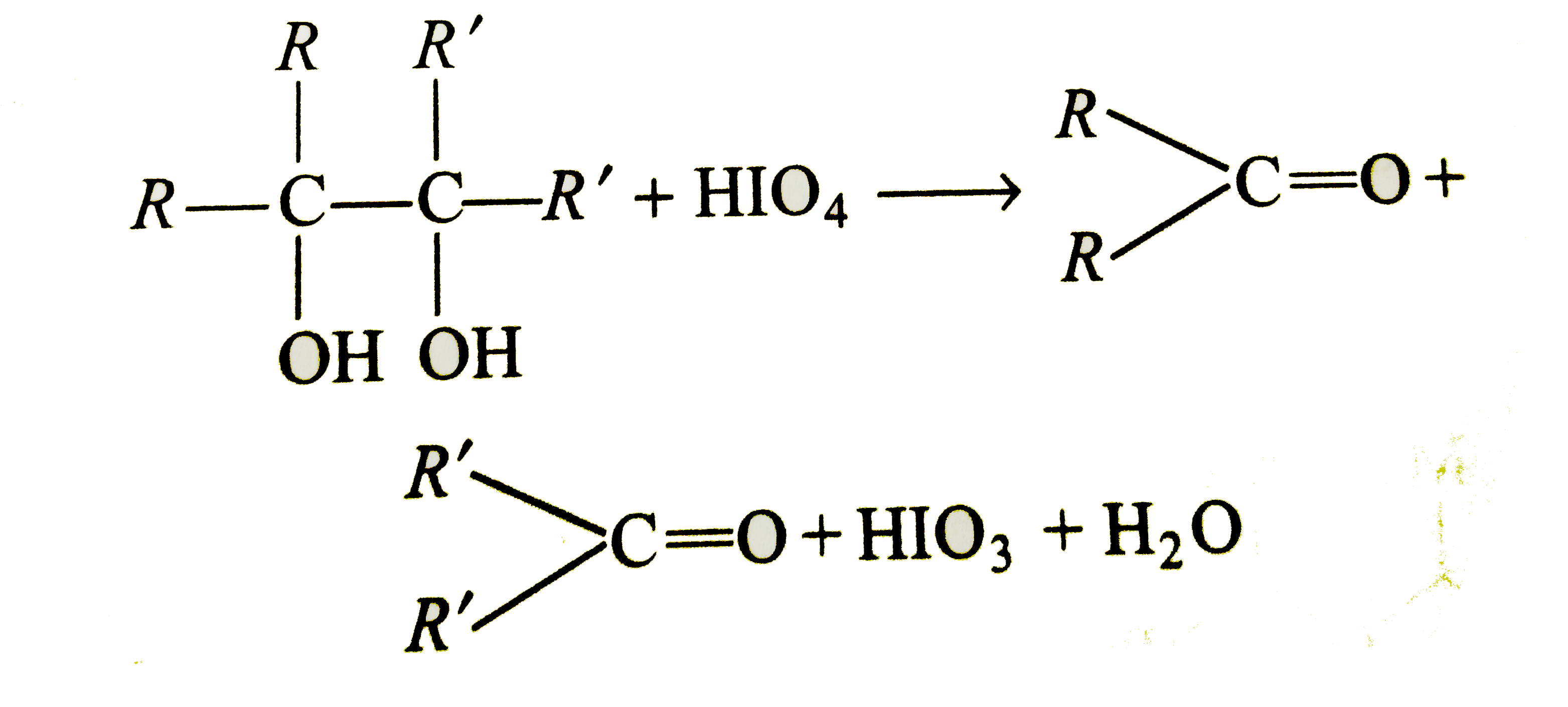
Cyclic diols react to give dicarbonyl compounds. The reactions are faster when the hydroxyl gorups are cid than when they are trans but both stereoisomers are oxidised by periodic acid. Periodic acid cleavage of vicinal diols is often used for analytic purposes as an aid in structure determination. By identifying the carbonyl compounds produced, The constitution of the diol may be deduced.
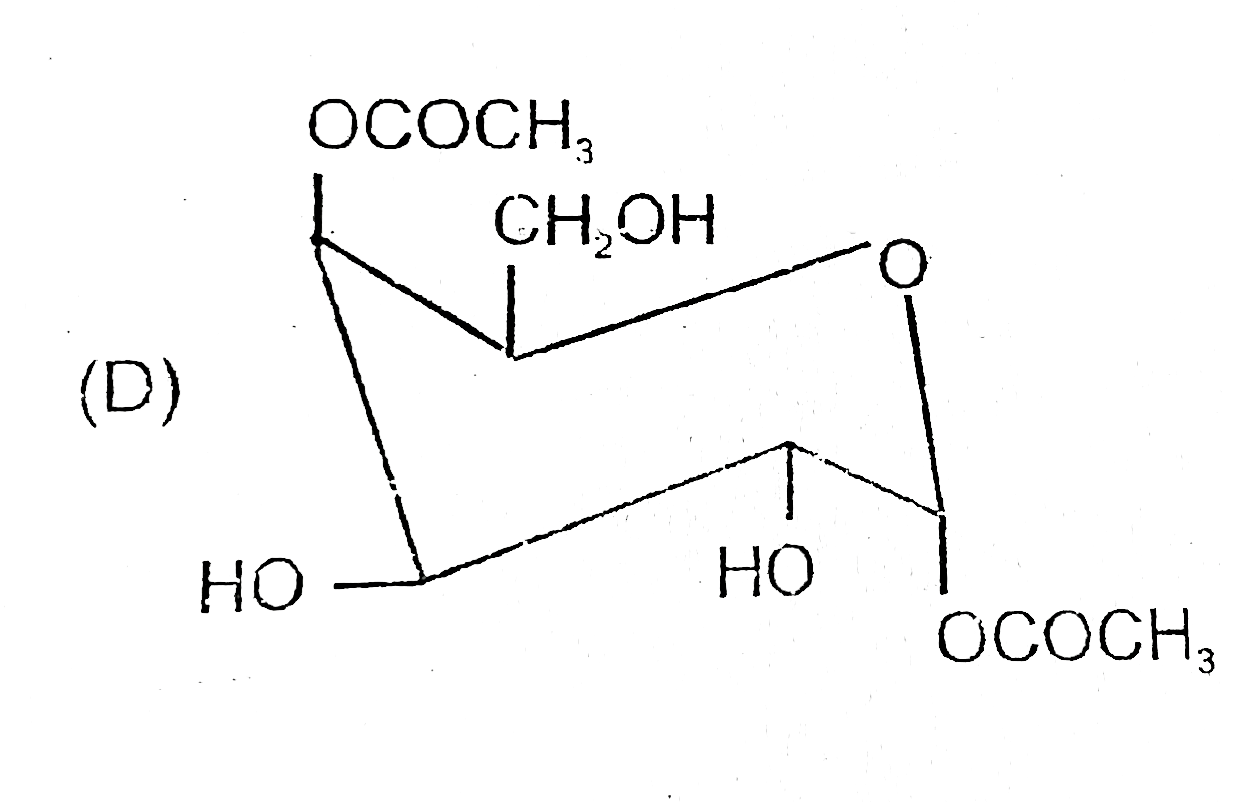
Which of the following compounds (s) is / are obtained when the following compound is oxidixed by HIO4 ?
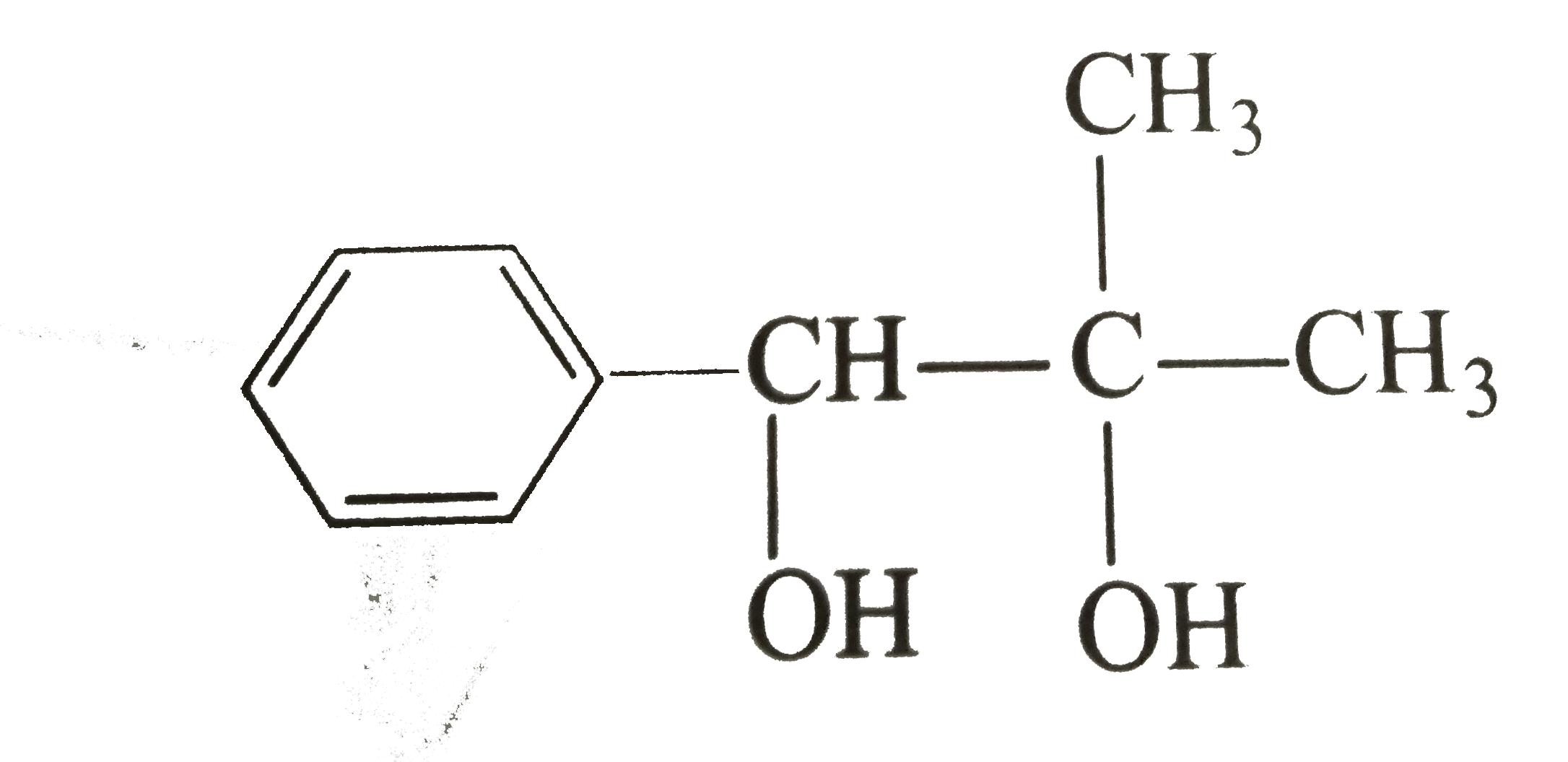
View SolutionAn amino acid with a phenolic hydroxyl group is
View SolutionThe number of hydroxyl groups in pyrophosphoric
View Solution