A subtance undergoes first order decomposition involving two parallel first order reaction as :
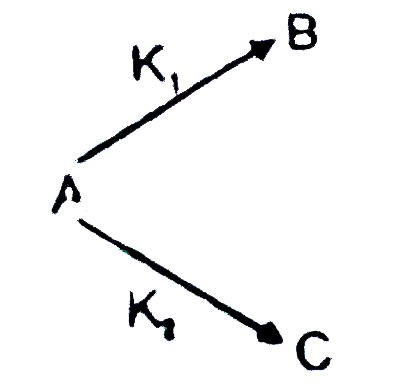
The mole percent of B in the products is :
A
23.17
B
76.68
C
30.16
D
69.84
Video Solution
Text Solution
The correct Answer is:2
k1×100k1+k2=1.28×10−41.25×10−4+3.80×10−5=76.83
|
Similar Questions
Knowledge Check
for first order reaction
A−k=2.303tlog(a−x)aBλ=2.303tlogNtN0C−K=2.303tlogaa−xDλ=2.303tlogaa−xFor a first order reaction
At0.75=4t0.5Bt0.75=2t0.5Ct0.75=3t0.5Dt0.75=1.5t0.5For a first order reaction
Athe degree of dissociation is equal to (1−e−kt)Ba plot of reciprocal concentration of the reactant vs. time gives a straight lineCthe time taken for the concentration of 75% reaction is thrice to t1/2 of the reactionDthe pre-expoential factor in the Arrhenius equation has the dimensions of time−1

