In the given isotherm select the incorrect statement?
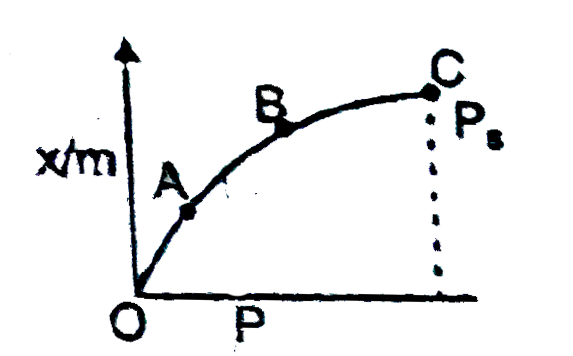
x/m∝P1/n along OA
x/m∝P∘ when ppoint B reached
x/m does not increase as rapidly with pressure along BC due to less surface area available for adsorption
Nature of isotherm is different for two gases for same adsorbent.
The correct Answer is:2
Similar Questions
A group between x/m and the presure P of the gas at a constant temperature is called adsorption siotherm. Where x is the no. of moles of the adsorbate and m is the mass of the adsorbent. adsoption isotherms of different shaopes have been experimentally observed .. According to frundlich adosroption isthem,
x/m=KP1/n
where K and N are constant paraments depending upon the nature of the solid and gas
Inn the given isotherm select the incorrect statement :
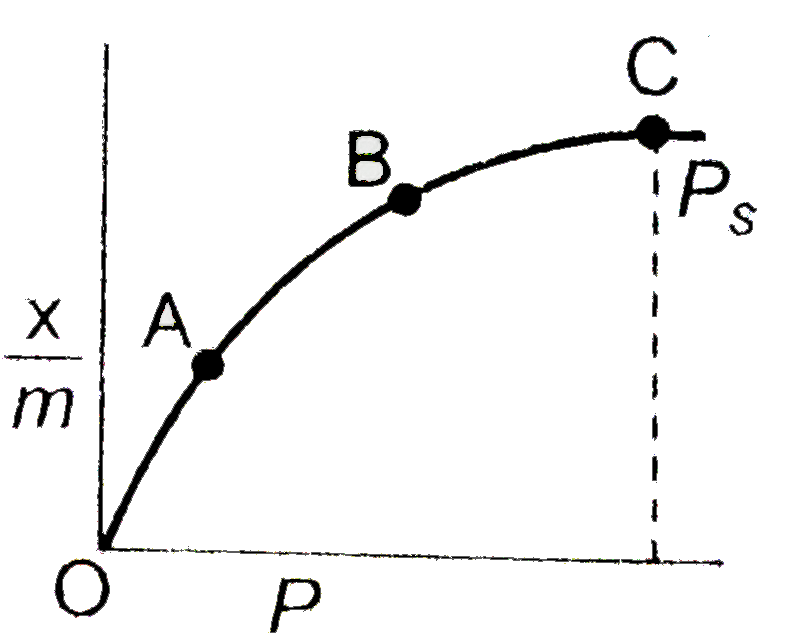
View SolutionA group between x/m and the presure P of the gas at a constant temperature is called adsorption siotherm. Where x is the no. of moles of the adsorbate and m is the mass of the adsorbent. adsoption isotherms of different shaopes have been experimentally observed .. According to frundlich adosroption isthem,
x/m=KP1/n
where K and N are constant paraments depending upon the nature of the solid and gas
Inn the given isotherm select the incorrect statement :

View Solution
Knowledge Check
Select the incorrect statements
AThe geometry of phosphorus in H3PO2,H3PO3 and H3PO34 is tetrahedralBH3PO2,H3PO3 and H3PO4 are tribasic acidsCNH3<PH3>AsH3<SbH3: increasing acidic character.DCO2<SiO2<SnO2<PbO2: increasing oxidising power.Select the incorrect statements :
AThe geometry of phosphorus in H3PO2,H3PO3 and H3PO34 is tetrahedralBH3PO2,H3PO3 and H3PO4 are tribasic acidsCNH3<PH3>AsH3<SbH3: increasing acidic character.DCO2<SiO2<SnO2<PbO2: increasing oxidising power.Select an incorrect statement
Alow temperature inactivates photosyntheticBdifferent habitats shows same response of phtosynthesis to a given tempertureCaffinity of the enzyme rubisCO for CO2 is reduced at higher temperatureDphotosynthesis is senitive to higher emperature primarily because of the fact that at higher temperatures enzymes becoms inactive

