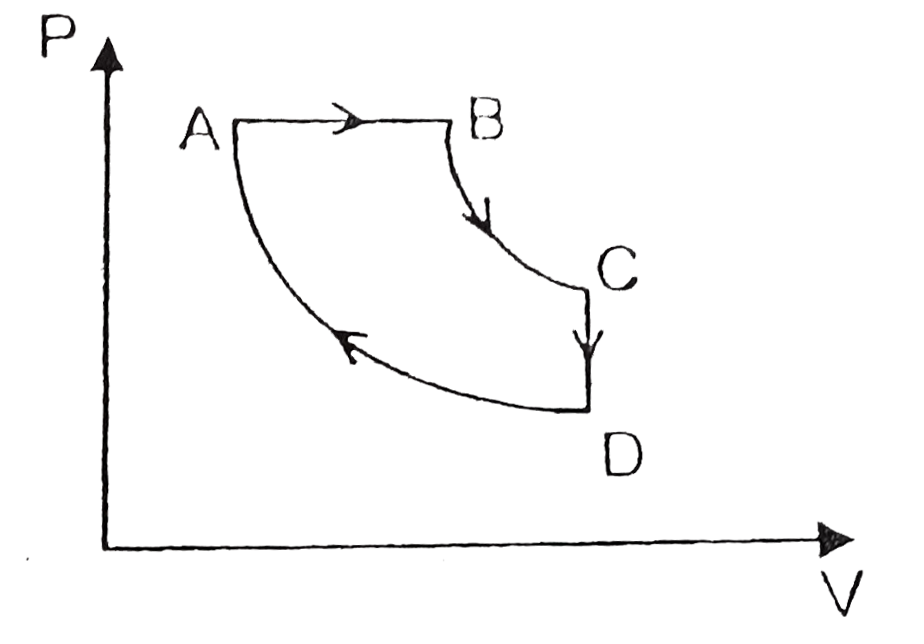
As shown in figure process AB is isobaric, BC is adiabasic CD is isochoric and DA is isothermal, through which n-moles of monoatomic gas undergo a cyclic process. The max & min temperature in the cycle are 4T0 and T0 respectively.
TB is greater than TD
Heat is released by gas in the process CD
Heat is supplied to the gas in the process AB
TC is greater than TA
The correct Answer is:A, B, C, D
DA is isothermal
∵TD=TA
TαPV
from graph (PV)C>(PV)D
BC is adiabatic expansion
so, TB>TC
so TB>TD and TB>TA
maximum temperature at B or C and minimum temperature at D or A.
TB=4TD
TD=TA=TB
IN process CD:ΔW=0,ΔQ=ΔU
pressure is decreasing, volume is constant. Temperature and intenal energy will decrease.
ΔQ=−ve, heat is released in CD.
In AB,ΔQ=nCPΔT
Volume is increasing and pressure is constant, temperature will increase ΔQ is positive so, heat is supplied to the gas in process AB.
Similar Questions
Knowledge Check
n moles of a monoatomic gas undergoes a cyclic process ABCDA as shown. Process AB is isobaric, process BC is adiabatic process CD is isochoric and process DA is isothermal the maximum temperature and minimum temperature in cycle are 4T0 and T0 respectively. Then:ATB>TC>TDBheat is released by the gas in the process CDCgeat is supplied by the gas to the surrounding in the process ABDheat is supplied to the gas to the surrounding in the process AB
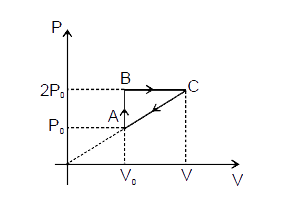
n moles of a diatomic gas has undergone a cyclic process ABC as shown in figure. Temperature at A is T0. Find
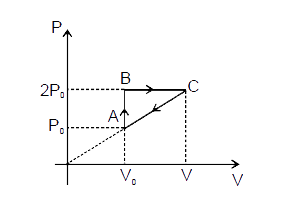
Maximum temperatue ?
View Solutionn moles of a diatomic gas has undergone a cyclic process ABC as shown in figure. Temperature at A is T0. Find
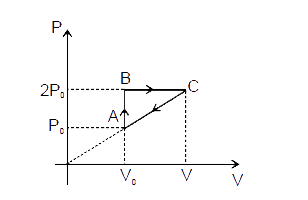
Find out the efficiency
View SolutionOne mole of a monoatomic gas behving ideally is used as
working substance in an engine working in the cycle as shown
in the figure . The process AB, BC, CD and DA are respectively
reversible isobaric, adiabatic isochoric and isothermal . The ratio
of maximum to volume and temperature during the
cycle is 8√2 and 4 respectively. If the maximum T is 800 K
and γ=5/3. Calculate ΔE (in kJ) for the process BC.
Given R=8.3J/K−mol.
View SolutionOne mole of monoatomic gas taken through a cyclic process as shown in figure.
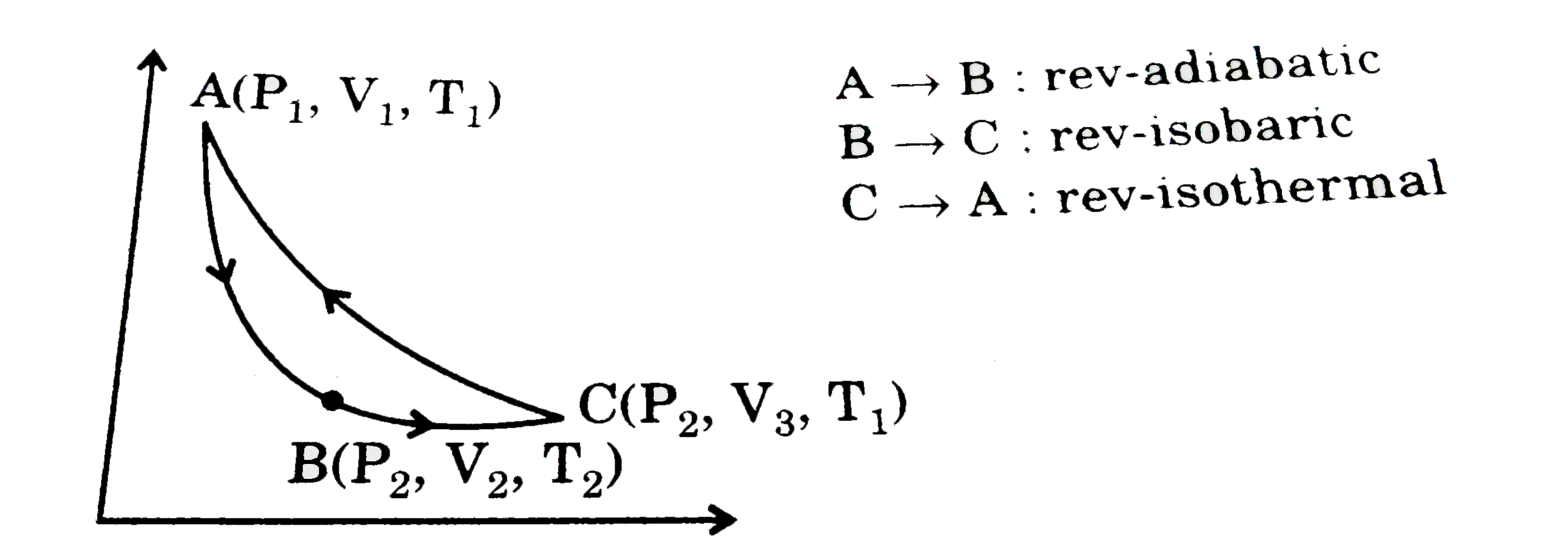
Calculate ∑ABCA=dqrevT.
View SolutionTwo moles of a monatomic ideal gas undergo a cyclic process ABCDA as shown in figure. BCD is a semicircle. Find the efficiency of the cycle.
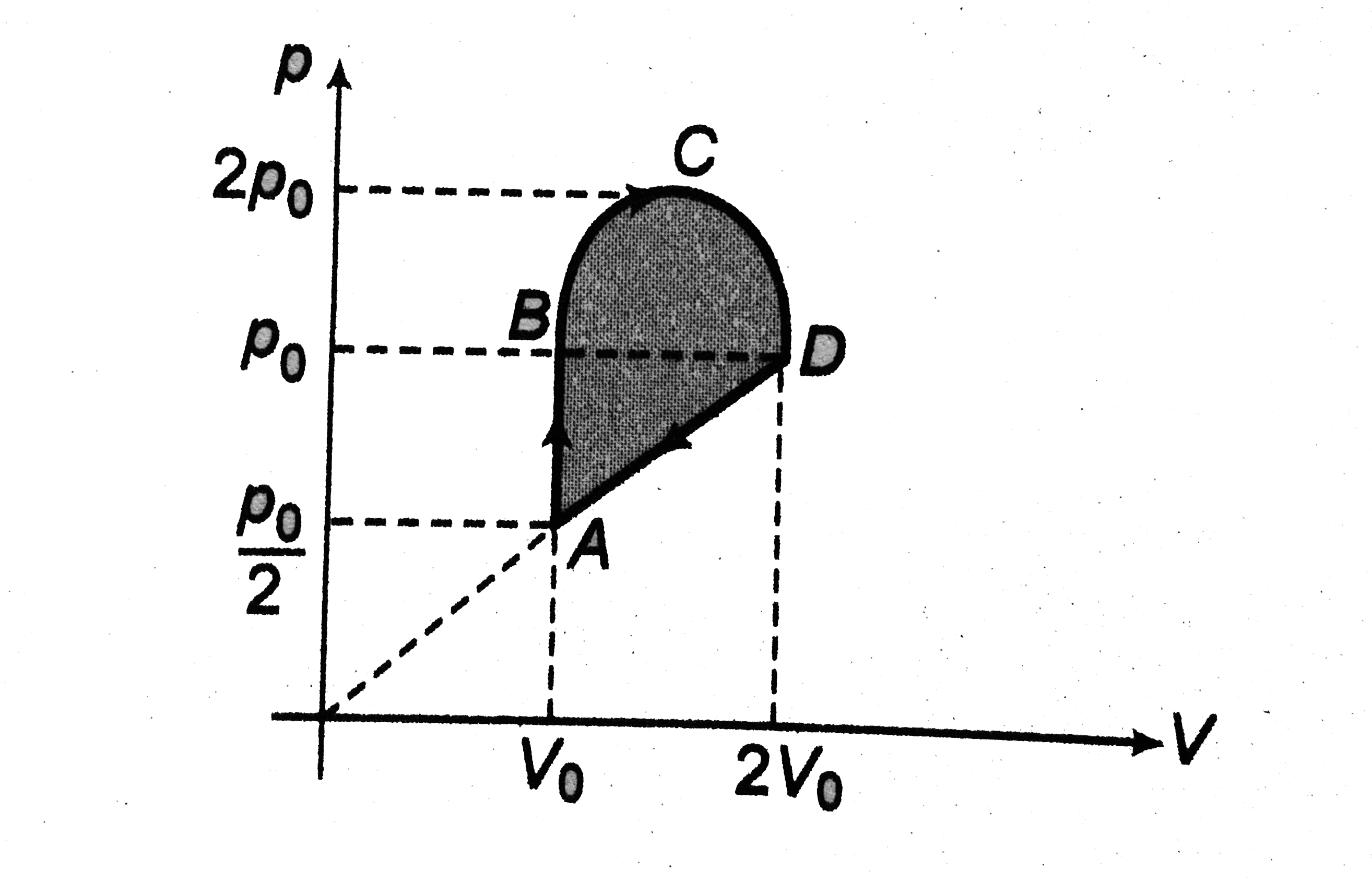
View SolutionOne mole of monoatomic has was taken through a cyclic process as shown in figure.
CalculatedqrevT
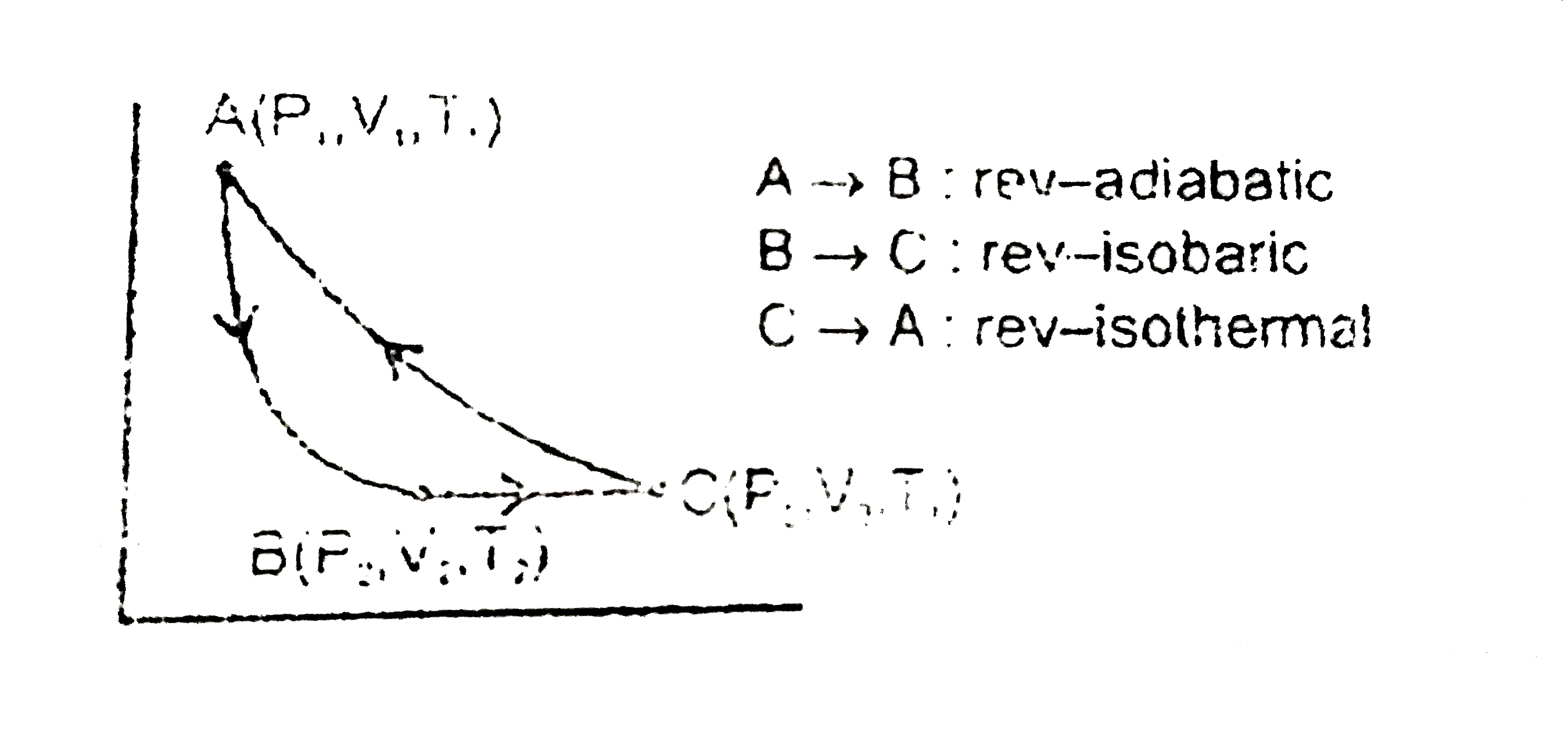
View SolutionTwo moles of a monoatomic ideal gas undergoes a process AB as shown in the figure. Then, find the work done during the process in Joule.
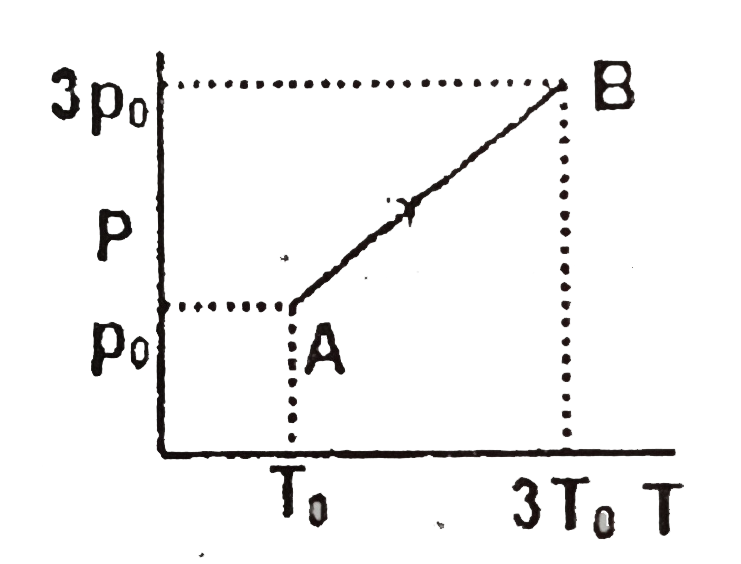
View Solution

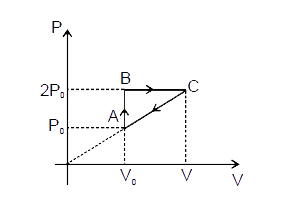 Total heat given to gas ?
Total heat given to gas ?