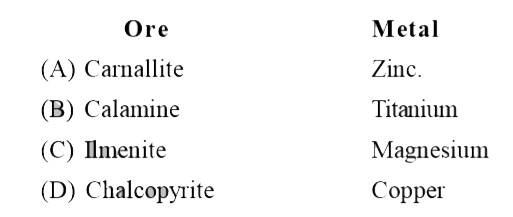Which of the following complexes are not correctly matched with the hybridisation of their central metal ions ?
A
[Ni(PPh3)3Cl]=dsp2
B
[Cu(CN)4]3−=sp3
C
[Co(H2O)6]3+=d2sp3
D
[Ni(NH3)6]2+=sp2d2
Video Solution
Text Solution
The correct Answer is:C
[Ni(PPh3)3Cl], in the formation of [Ni(PPh3)3Cl] nickel atom undergoes sp3 hybridization, hence it is tetrahedral in shape.

|
Similar Questions
Knowledge Check
Which of the following complexes are not correctly matched with the hybridisation of their central metal ion ?
(a) [Ni(CO)4] , sp3 (b) [Ni(CN)4]2− , sp3
(c ) [CoF6]3− , d2sp3 (d) [Fe(CN)6]3− , sp3d2
Select the correct option :A(i) and (ii)B(i) and (iii)C(ii) and (iv)D(ii), (iii) and (iv)Which of the following complexes are not correctly matched with the hybridisation of their central metal ion ?
(a) [Ni(CO)4] , sp3 (b) [Ni(CN)4]2− , sp3
(c ) [CoF6]3− , d2sp3 (d) [Fe(CN)6]3− , sp3d2
Select the correct option :A(i) and (ii)B(i)and (iii)C(i) ,(iii) and (iv)D(ii), (iii) and (iv)Which of the following complex is not correctly matched with its geometry ?
A[NiCl2(Ph3P)2]−tetrahedralB[Co(Py)4]2+−square planarC[Cu(CN)4]3−−tetrahedralD[Fe(CO)4]2−−square planar
Which of the following metal is correctly matched with its ore :

View SolutionWhich of the following complexes is correctly matched with their geometrics ?
View SolutionWhich of the following complex is not correctly matched with its geometry ?
View SolutionWhich of the following complexes is correctly matched with their geometrics ?
View SolutionWhich of the following complex (s) is/are correctly matched with their geometry ?
View Solution