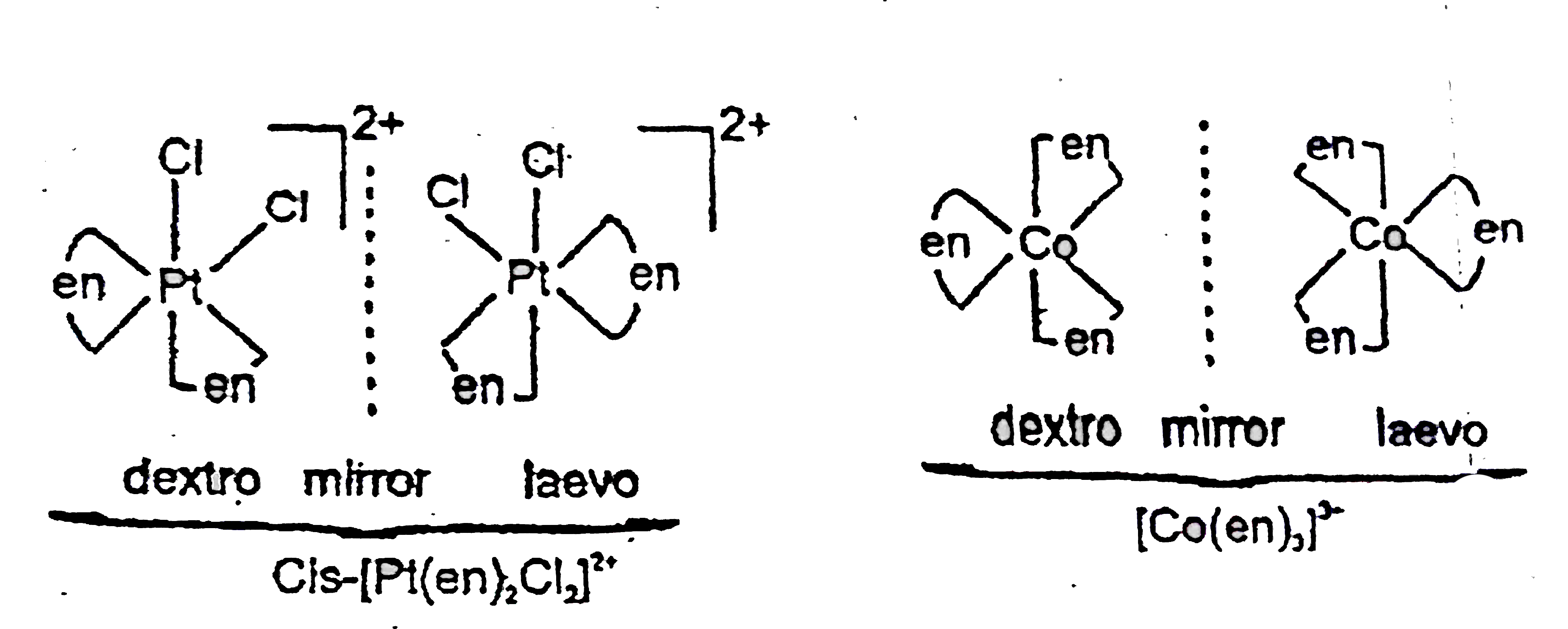In which of the following pairs both the complexes show optical isomerism?
A
Cis−[CrCl2(OX)2]2−,trans−[CrCl2(OX)2]3−
B
[Fe(NH3)2(CN)4]−[Fe(en)3]3+
C
Cis−[Pt(en)2Cl2]2+,[Co(en)3]3+
D
Cis−[Co(NH3)4(NO2)2]+,cis(Cr(gly)3]
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:RESONANCEChapter:COORDINATION COMPOUNDSExercise:Miscellneous Solved Problem (MSPS)
Explore 20 Videos
- COORDINATION COMPOUNDSBook:RESONANCEChapter:COORDINATION COMPOUNDSExercise:Exercise Part-I: Subjective Question Section (A)
Explore 5 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:RESONANCEChapter:CHEMISTRY IN EVERYDAY LIFEExercise:ORGANIC CHEMISTRY(Chemistry in every day life)
Explore 31 Videos
- D & F BLOCK ELEMENTSBook:RESONANCEChapter:D & F BLOCK ELEMENTSExercise:INORGANIC CHEMISTRY(d & f- Block Elments)
Explore 40 Videos
Similar Questions
Knowledge Check
In which of the following pairs both the complex show optical isomerism? .
Aa)cis−[Cr(C2O4)2CI2]3−,cis−[Co(NH3)4CI2]Bb)[Co(en)3]CI3,cis−[Co(en)2CI2]CICc)[Co(NO3)3(NH3)3],cis−[Pt(en)2CI2]Dd)[PtCI(en)CI],[NiCI2Br2]2−In which of the following pairs both the complexes show geometrical isomerism?
A[Cr(en)2Cl2]+,[Cr(en)3]3+B[Co(NH3)4Cl2]+,[Co(en)2Cl2]+C[Pt(NH3)(H2O)Cl2],[Co(NH3)2(NO2)](NO3)2D[Fe(NH3)2]+,[Fe(NH3)(CN)5]2−In which of the following pairs both the complexes do not show optical isomerism ?
Acis−[Cr(en)2Cl2] and cis−[Co(NH3)4Cl2]B[Co(gly)3] and cis−[CoCl2(en)2]C[Pt(en)Cl2] and fac−[Ni(NH3)3Cl3]Dmer−[Co(NO3)3(NH3)3] and [PtCl2(en)]
Which of the following is most likely to show optical isomerism ?
View SolutionWhich of the following is most likely to show optical isomerism ?
View SolutionWhich of the following is most likely to show optical isomerism?
View SolutionWhich one of the following complexes shows optical isomerism ?
View SolutionWhich one of the following complexes shows optical isomerism ?
View Solution