Which of the following statement is correct ?
A
Shine of freshly cut sodium is due to strong metallic bonding
B
Rb2CO3 decomposes readily on heating to RbO and CO2.
C
Among hydroxides of alkali metals, LiOH is least soluble whereas CsOH is highly soluble in water.
D
White metal is an alloy of lithium with zinc.
Video Solution
Text Solution
The correct Answer is:3
(1) It is due to oscillations of free / mobile electrons.
(2)Rb2CO3 does not decomposes as it has very which thermally stability smongst the alkali metal carbonates on account of the fact that higger cation stabilises the bigger anion through crystal lattice effect.
(3) Down the alkali metal group, the solubility of the hydroxides increases as there is more decrease in lattice energy as compared to that of hydration energy.
The order is thus, CsOH>RbOH>KOH>NaOH>LiOH.
(4) White metal is an alloy of lithium with lead.
|
Similar Questions
Knowledge Check
Which of the following statements is correct ?
ASome tranquilizers function by inhibiting the enzymes which catalyse the degradation of noradrenaline.BTranquilizers are narcotic drugs.CTranquilizers are chemical compounds that do not effect the message transfer from nerve to receptor .DTranquilizers are chemical compounds that can relieve pain and fever.Which of the following statements is correct?
ACommunicator should have excellent sensesBCommunicator should have acceptance powerCCommunicator should be soft spokenDCommunicator should have good personalityWhich of the following statements is correct?
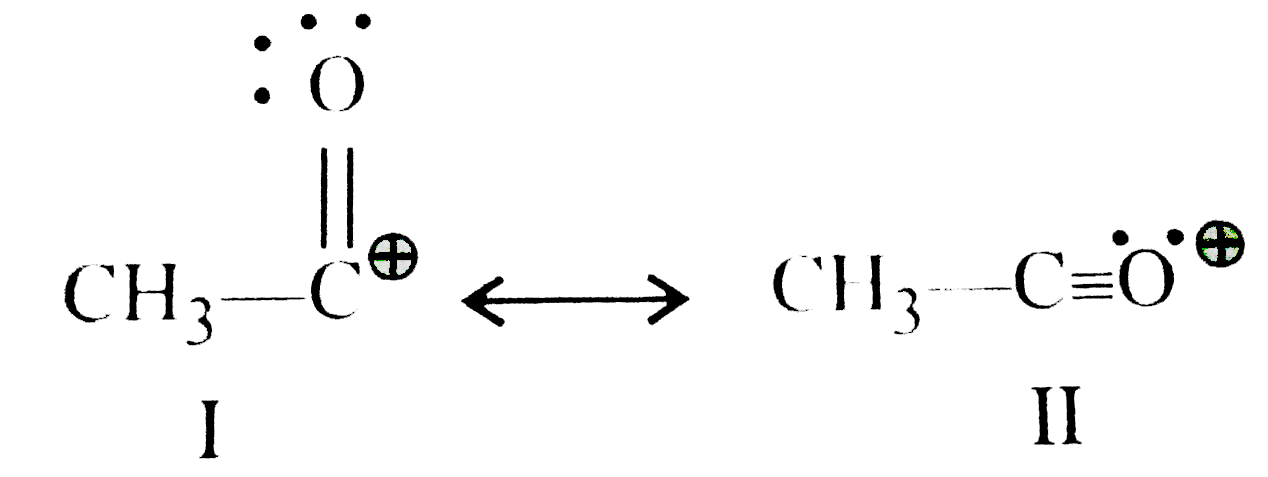 ADipole moment :
ADipole moment :
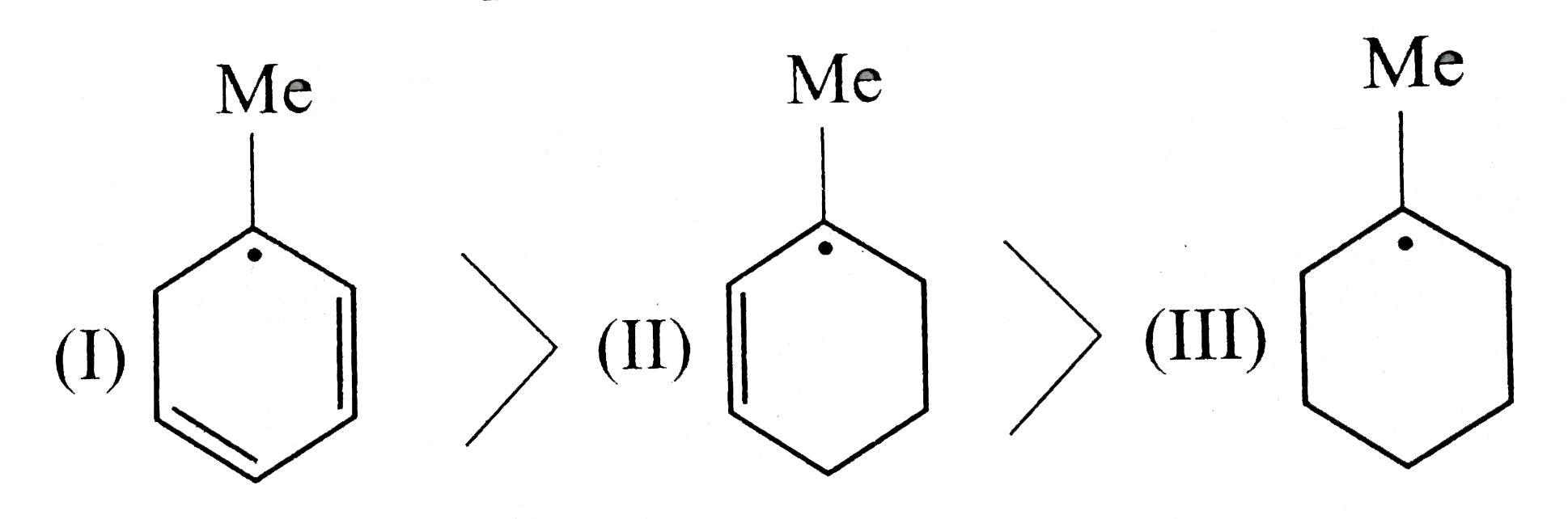
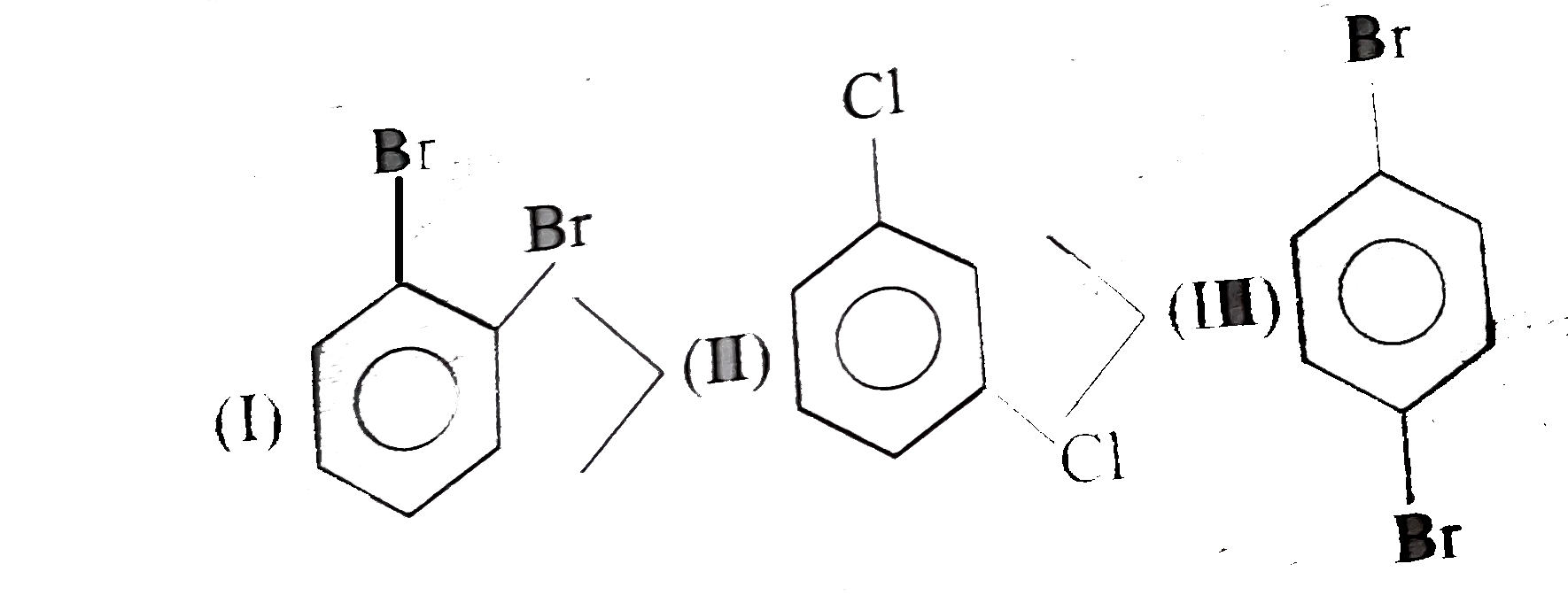 BStability of free radical :
BStability of free radical :
 CBasic strength : CH3OӨ>ӨOH>RSӨDBasic and nucleophilic strength : IӨ>BrӨ>C1Ө>FӨ
CBasic strength : CH3OӨ>ӨOH>RSӨDBasic and nucleophilic strength : IӨ>BrӨ>C1Ө>FӨ

