Give the chemical reaction to support that +5 oxidation state of Bi is less stable than +3 state.
Video Solution
Text Solution
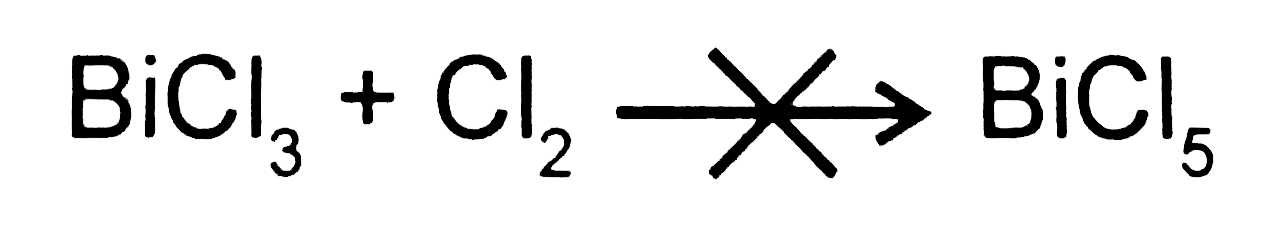
Due to inert pair effect, Bi can show +3 and +5 oxidation states. Since the inert pair effect is maximum in case of Bi, therefore, its +5 oxidation state is less stable than +3 oxidation state.This is evident from the observation that BiCl3 even on prolonged heating with Cl2 does not form BiCl5
|
Topper's Solved these Questions
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - Exercise 1 part-1
14 Videos
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - Exercise 1 part-2
32 Videos
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - ALP PART OBJECTIVE
12 Videos
- NUCLEAR CHEMISTRYBOOK - RESONANCECHAPTER - NUCLEAR CHEMISTRYEXERCISE - STAGE-II
1 Video
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)BOOK - RESONANCECHAPTER - P-BLOCK ELEMENT (BORON AND CARBON FAMILY)EXERCISE - PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
8 Videos
Similar Questions
Knowledge Check
The oxidation state of -3 is least stable in
AAsBPCBiDSbStable oxidation state of No is
A+2B+3C+4D+7Stable oxidation state of Yb is
A+2B+3C+4D+6

