An inorganic iodide (A) on heating with a solution of KOH gives a gas (B) and a solution of a compound. The gas (B) on ignition in air gives a compound (C ) and water. Copper sulphate is finally reduced to the methal on passing (B) through its solution.
What is true about gas (B) and compound (C ) ?
The oxidation number of central atom of gas (B) is +IV
The gas (B) produces a black precipitate of methallic sliver with sliver nitrate solution.
Compound (C ) dissolves in water forming an acid which with solution hydroxide forms three series of salts.
(B) and (C ) both
(A)isPH4I. "The given changes are" :
(i) P(A)H4I+KOH→KI+PH3(B)+H2O
(ii) 4PH3(B)+8O2→P4(C)O10+6H2O
(iii) 4Cu++PH3+4H2O→H3PO4+4Cu⏐↓+8H+
3AgNO3+PH3→Ag3P↓+3HNO3
Ag3P+3agNO3+3H2O→6Ag⏐↓(black)+3HNO3+H3PO3
6Ag++PH3+3H2O→3H3PO3+6Ag⏐↓(black)+6H+
In P4O10 there are 16σ−bonds and all central atoms have sp3 hybridisation.
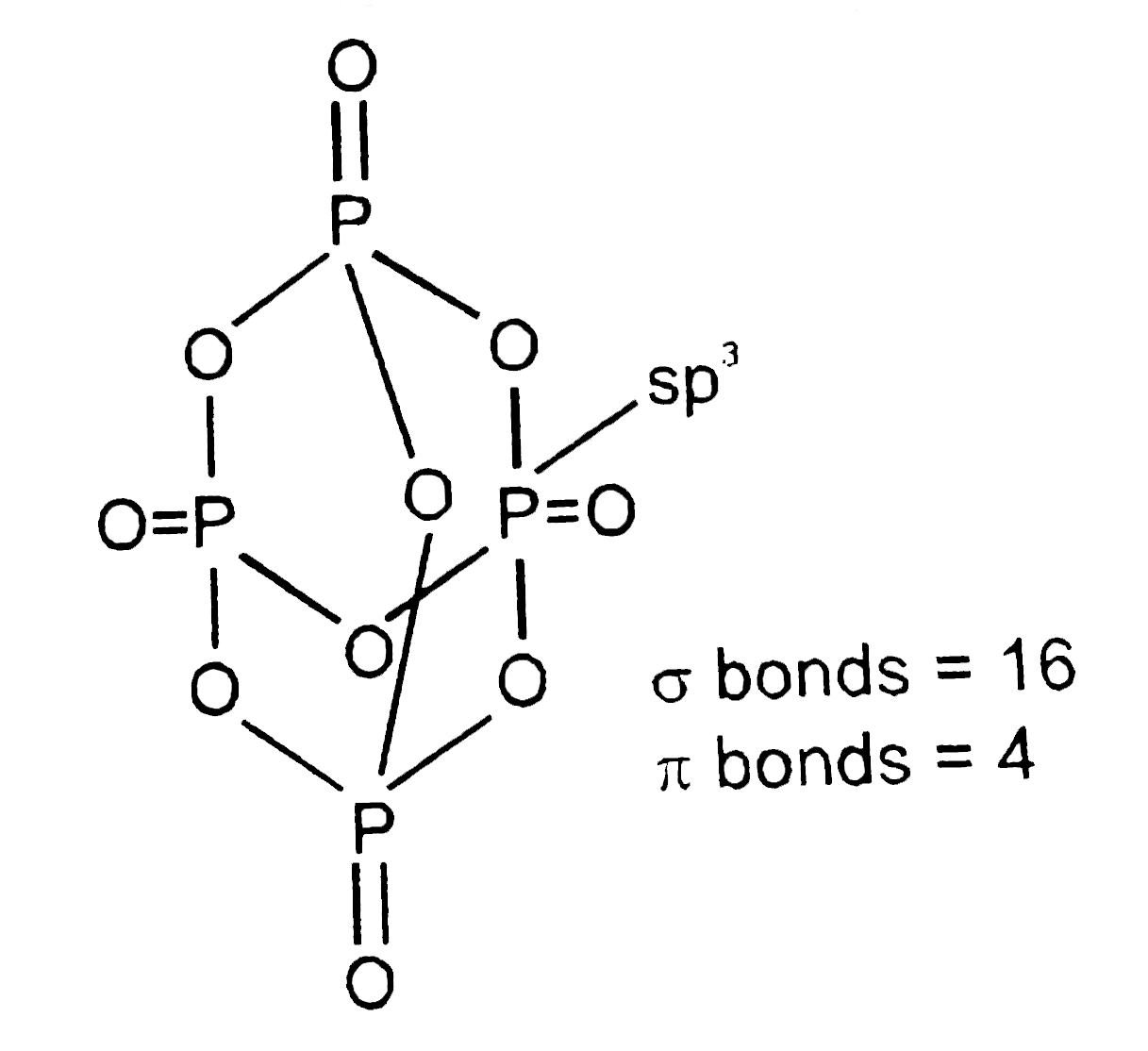
Similar Questions
An inorganic compound 'A' on heating with solution of KOH, gives a gas B and the solution of compound C. gas B on ignition with excess of air gives a compound D and water. Copper sulphate gives a black precipitate on passing through its solution. White precipitate E is obtained on reaction of C with copper sulphates solution.
Compound D is:
View SolutionIdentify (A) to (E).
(a) An inorganic iodide (A) on heating with a solution of KOH gives a gas (B) and the solution of a compound (C).
(b) The gas (B) on ignition air gives a compound (D) and water.
( C) Copper sulphate is reduced to the metal on passing (B) through the solution.
(d) A precipitate of the compound (E) is formed on reaction of (C) with copper sulphate solution.
View SolutionThe compound (X) on heating gives a colourless gas a nd a residue that is dissolved in water to obtain (B). Excess of CO2 is bubbled through aqueous solution of B, C is formed. Solid (C) on heating gives back X. (B) is
View Solution(i) An inorganic iodide (A) on heating with a solution of KOH gives a gas (B) and the solution of a compound ( C).
(ii) The gas (B) on ignition in air gives a compound (D) and water.
(iii) Copper sulphate forms a black precipitate on passing (B) through its solution.
(iv) A precepitate of compound (E) is formed on reaction of (C) with copper sulphate solution.
Identify (A) to (E) and give chemical equations for reactions at steps (i) to (iv).
View SolutionThe compound 'A' on heating gives a colourless gas and a residue. The residue dissolves in water to obtain (B). When excess of CO_(2) is bubbled through aqueous solution of B gentle heating gives back A. Compound 'C' is
View SolutionMagnesium on heating in air gives (A) and (B). On reaction with water (B) gives a colourless gas (C).(C) when passed through CuSO4 solution, gives a blue coloured solution (D). Identify (C)
and (D).
View SolutionAn aqueous solution of salt (A) gives a give a white precipitate ( B) with sodium chloride solution . Compound ( B) dissolves in hot water and the solution on treatment with sodium iodide give a yellow precipitate ( D) ,. and on passing H_2 S through solution ( B) gives a black ppt . (C) . Compound (A) does not give any gas with dil HCl , but liberates a reddish brown gas on heating identify compounds (A), (B), ( C) , and (D) .
View SolutionIdentify (A) to ( E) . (a) An inorganic iodide (A) on heating with a solution of KOH gives a gas (B) and the solution of a compound ( C) . (b) The gas (B) on ignition air gives a compound (D) and water. ( C) Copper sulphate is reduced to the metal on passing (B) through the solution. (d) A precipitate of the compound ( E) is formed on reaction of ( C) with copper sulphate solution.
View Solution
