(a) What amount of CaO in grams is required to neutralise 852g of P4O10.
(b) Write the structure of P4O10
(b) Write the structure of P4O10.
Video Solution
Text Solution
The correct Answer is:A, B, C
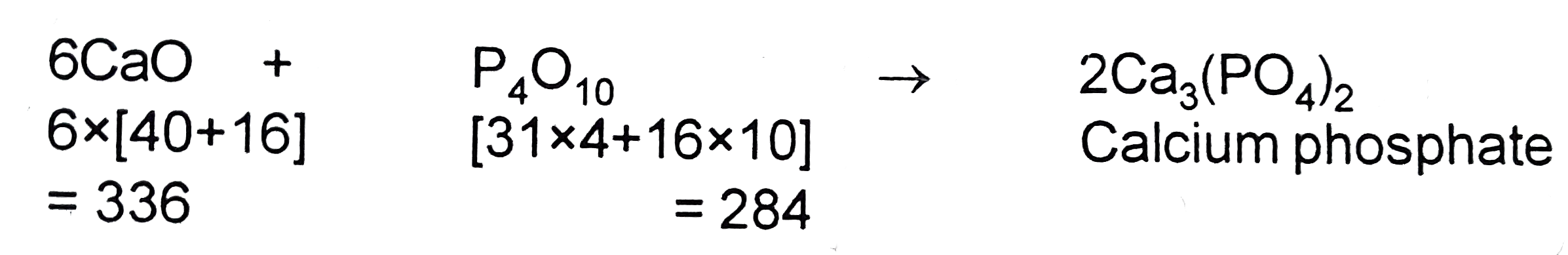
According to above reaction,
284g of P4O10 are completley recacted with 336g of CaO.
∴852g of P4O4"will react with" 336×852284g "of" CaO.
Hence, required weight of CaO = 336×852284=1008g
|
Topper's Solved these Questions
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - ALP PART II AIEEE OBJECTIVE
1 Video
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - ALP PART 2 OBJECTIVE
5 Videos
- P BLOCK ELEMENTSBOOK - RESONANCECHAPTER - P BLOCK ELEMENTSEXERCISE - ALP EX #3 OBJECTIVE
1 Video
- NUCLEAR CHEMISTRYBOOK - RESONANCECHAPTER - NUCLEAR CHEMISTRYEXERCISE - STAGE-II
1 Video
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)BOOK - RESONANCECHAPTER - P-BLOCK ELEMENT (BORON AND CARBON FAMILY)EXERCISE - PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
8 Videos
Similar Questions
Knowledge Check
In P4O10,
Asecond bond in P=O is formed by pπ−dπ back bondingBP=O bond is formed by pπ−pπ bondingCP=O bond is formed by dπ−dπ bondingDP=O bond is formed by dπ−dπ−3σ back bondingP4O10 is the anhydride of
AH3PO2BH3PO3CH3PO4DH4P2O7P4O10 is the anhydride of
AH3PO2BH3PO3CH3PO4DH4P2O7
How many grams of CaO are required to neutralise 852 g of P4O10?
View SolutionHow many grams of CaO are required to neutralise 852 g of P4O10 ? Draw structure of P4O10 molecule.
View SolutionHow many grams of CaO are required to neutralise 852g of P4O10 ? Draw the structure of P4O10.
View SolutionCalculate the amount of calcuium oxide required when it reacts with 852g of P4O10.
View SolutionCalculate the amount of calcium oxide required when it reacts with 852g of P4O10.
View Solution

