The highly metallic will have the configuration of
A
2,8,7
B
2,8,8,5
C
2,8,81
D
2,8,2
Video Solution
Text Solution
The correct Answer is:C
NA
|
Similar Questions
Knowledge Check
A will have configurationA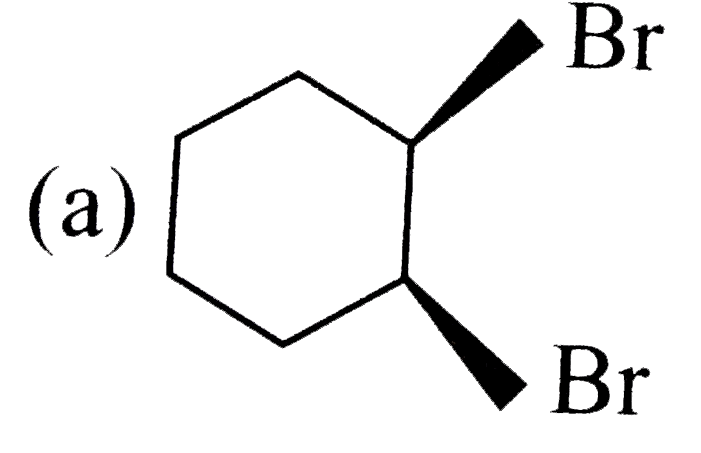 B
B Cboth are trueDNone of these
Cboth are trueDNone of theseThe lowest magnetic moment is shown by the transition metal ion with the configuration
A3d7B3d9C3d2D3d3Consider the following reaction,
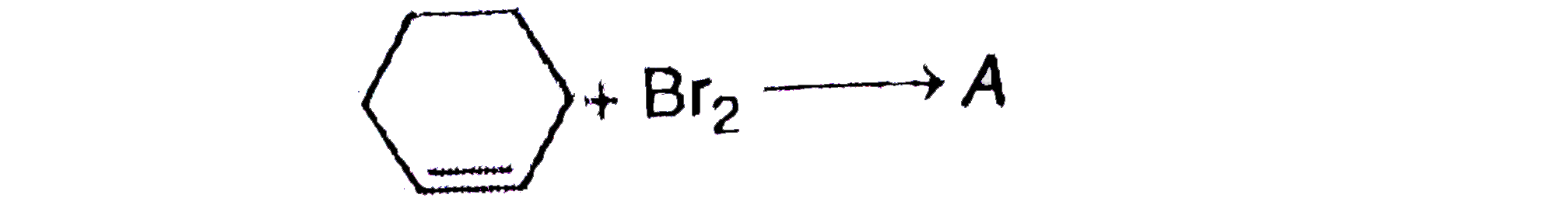
A will have configurationA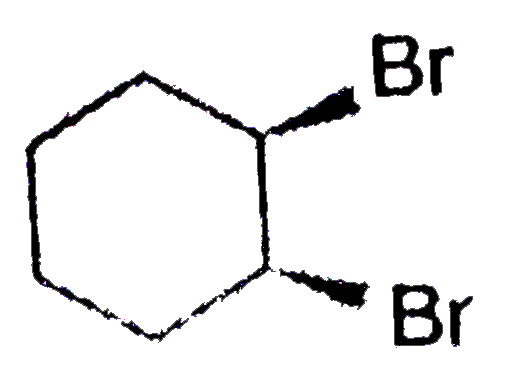 B
B CBoth (a) and (b)DNone of these
CBoth (a) and (b)DNone of these
Which of the following have same configuration.
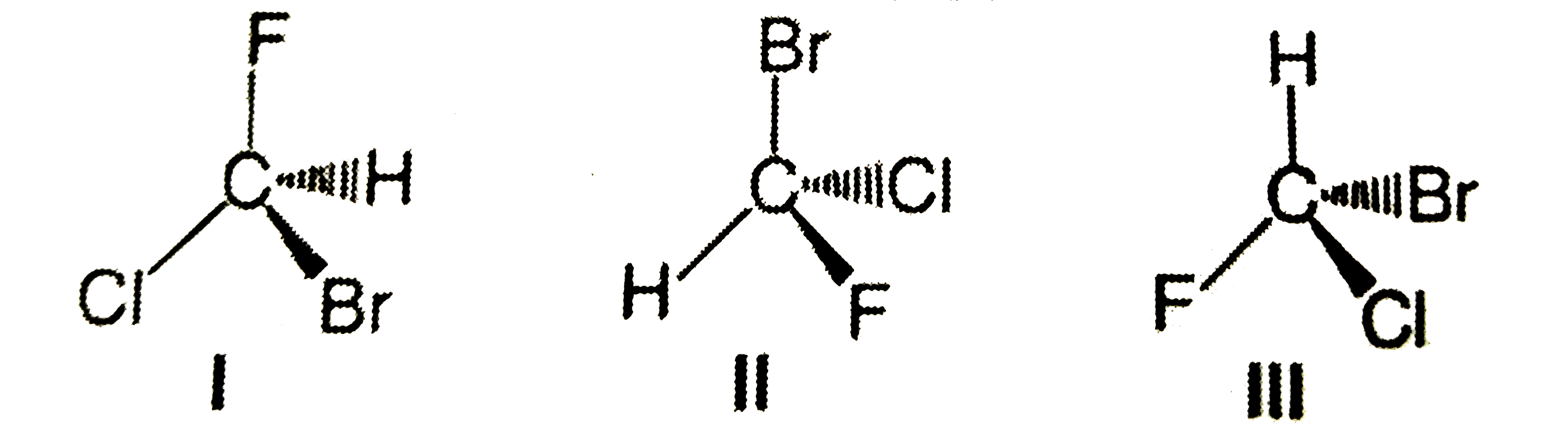
View SolutionAmong the following series of transition metal ions, the one where all metal ions have 3d2 electronic configuration is:
View SolutionAmong the following series of transition metals ions, the one where all metal ions have 3d2 electronic configuration is:
View SolutionThe metal which is considered as configuration of neutral titanium atom is
View SolutionWhich of the ions is not having the configuration of Ne?
View Solution