Would you expect the first ionisation enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
Video Solution
Text Solution
Ionization enthalpy among other things depends upon the electronic configuration ( number of electrons) and nuclear charge (number of protons). Since the isotopes of an element have the same electronic configuration and same nuclear charge they are expected to have same ionization enthalpy.
|
Similar Questions
Knowledge Check
Isotopes of the atoms of same element have -
Asame number of neutronsBsame number of protonsCsame atomic massDdifferent chemical propertiesIsotopes exist because atoms of the same element can have different numbers of
AprotonsBneutronsCelectronsDNone of the above.
Is the mass percent of a solution same as its strength ? Justify your answer.
View Solution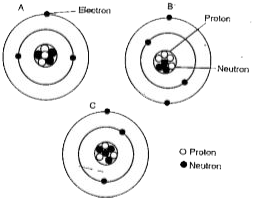
Which diagram represents,isotopes of the same element?
View SolutionWould you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer.
View SolutionWould you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer.
View SolutionWould you expect the second electron gain enthalpy of S as positive, more negative or less negative than the first? Justify your answer.
View SolutionAre polyesters and polyacrylates same ? Justify your answer.
View Solution
