Arrange the following ions in order of decreasing ionic radii :Li2+,He+,Be3+
To arrange the ions Li2+, He+, and Be3+ in order of decreasing ionic radii, we can follow these steps:
Step 1: Identify the number of electrons in each ion.
- Lithium ion (Li2+): Lithium has 3 electrons, and losing 2 gives us 1 electron.
- Helium ion (He+): Helium has 2 electrons, and losing 1 gives us 1 electron.
- Beryllium ion (Be3+): Beryllium has 4 electrons, and losing 3 gives us 1 electron.
Step 2: Determine if the ions are isoelectronic.
All three ions (Li2+, He+, and Be3+) have 1 electron, making them isoelectronic.
Step 3: Understand the relationship between charge and ionic radius.
For isoelectronic species, the ionic radius decreases with an increase in positive charge. This is because a higher positive charge pulls the electrons closer to the nucleus due to increased nuclear attraction.
Step 4: Compare the charges of the ions.
- He+ has a charge of +1.
- Li2+ has a charge of +2.
- Be3+ has a charge of +3.
Step 5: Arrange the ions based on their ionic radii.
Since He+ has the least positive charge, it will have the largest ionic radius. Conversely, Be3+ has the most positive charge and will have the smallest ionic radius. Therefore, the order of decreasing ionic radii is:
1. He+ (largest)
2. Li2+
3. Be3+ (smallest)
Final Answer:
The order of decreasing ionic radii is:
He+>Li2+>Be3+
- Lithium ion (Li2+): Lithium has 3 electrons, and losing 2 gives us 1 electron.
- Helium ion (He+): Helium has 2 electrons, and losing 1 gives us 1 electron.
- Beryllium ion (Be3+): Beryllium has 4 electrons, and losing 3 gives us 1 electron.
All three ions (Li2+, He+, and Be3+) have 1 electron, making them isoelectronic.
For isoelectronic species, the ionic radius decreases with an increase in positive charge. This is because a higher positive charge pulls the electrons closer to the nucleus due to increased nuclear attraction.
- He+ has a charge of +1.
- Li2+ has a charge of +2.
- Be3+ has a charge of +3.
Since He+ has the least positive charge, it will have the largest ionic radius. Conversely, Be3+ has the most positive charge and will have the smallest ionic radius. Therefore, the order of decreasing ionic radii is:
1. He+ (largest)
2. Li2+
3. Be3+ (smallest)
The order of decreasing ionic radii is:
He+>Li2+>Be3+
Similar Questions
Knowledge Check
Arrange the following ions in order of their inceasing radii : Na+,Mg2+,K+,Al3+
ANa+>Al3+>Mg2+>K+BMg2+<Al3+<Na+<K+CAl3+<Mg2+<Na+<K+DNa+<K+<Mg2+<Al3+Arrange the following in decreasing order of E2 reaction :
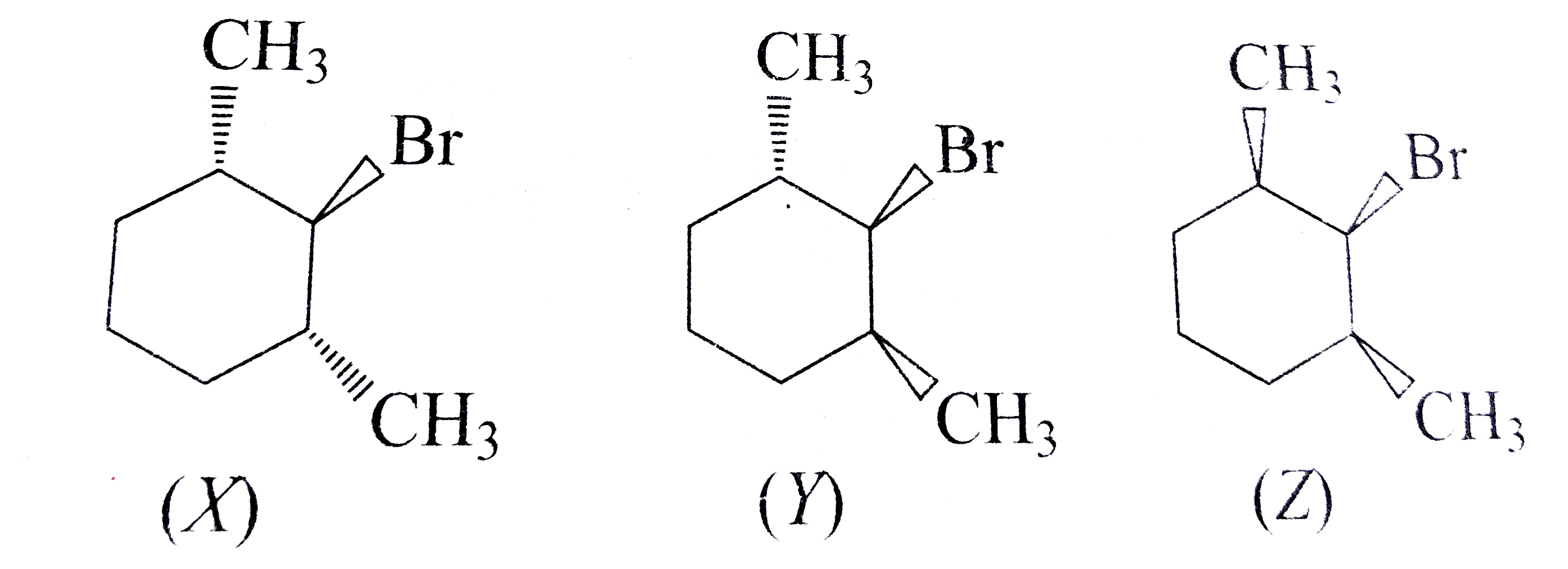 AX>Y>ZBX>Z>YCY>Z>XDZ>Y>X
AX>Y>ZBX>Z>YCY>Z>XDZ>Y>XArrange the following in decreasing order of acidity ?
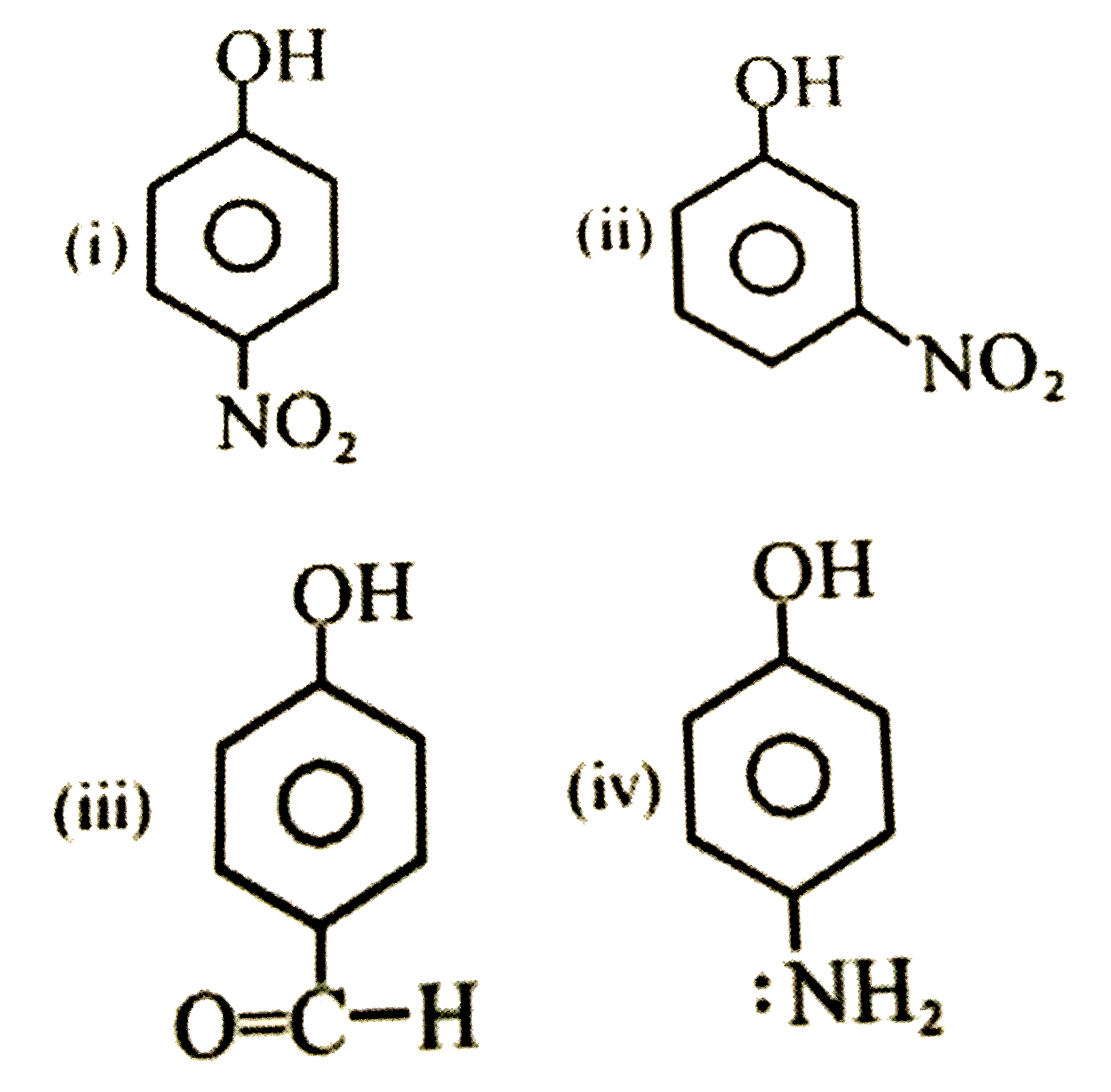 AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IV
AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IV
Arrange the following ions in order of increasing ionic radii.
Li+,Na+,Al3+,K+,Mg2+
View SolutionArrange the following ions in order of increasing ionic radii.
Li+,Na+,Al3+,K+,Mg2+
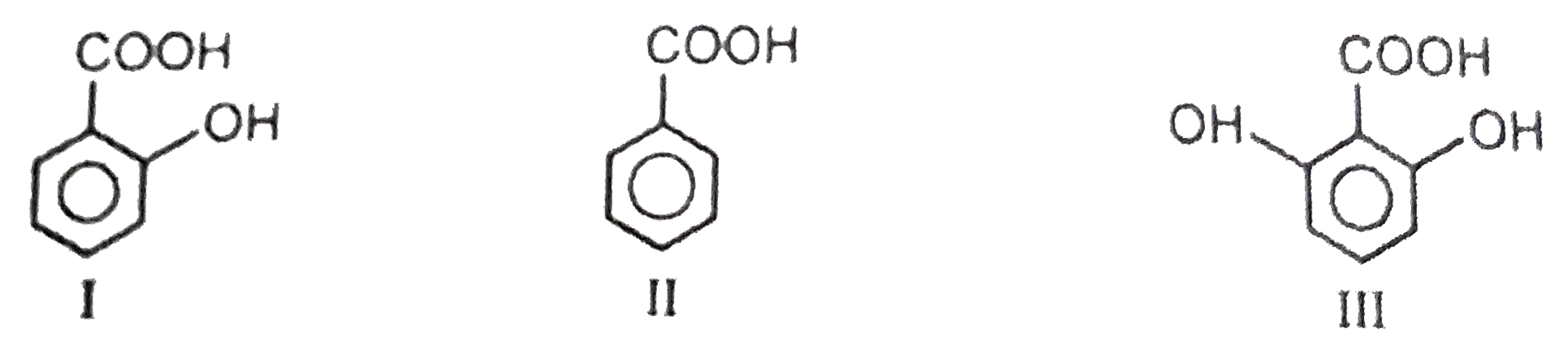
View SolutionArrange the following in decreasing order of acidity
View SolutionArrange the following in decreasing order of basicity:

View SolutionArrange the following in decreasing order of stability.
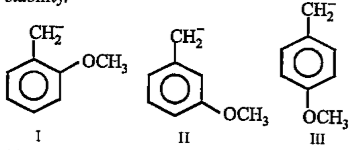
View Solution

