Although fluorine is more electronegative than chlorine but chlorine can be converted into chloride ion more easily as compared to fluoride ion from fluorine . Explain.
Video Solution
Text Solution
Since electron gain enthalpy of C1 is more negative than that of F, therefore more energy is released when
C1→C1− as compared to when F→F−
|
Similar Questions
Knowledge Check
Fluorine is more reactive than chlorine because
Afluorine has lower bond energyBfluorine has a greater ionisation potentialCthe covalent bond in fluorine molecule is weakerDfluorine has no available d-orbitals.Which chlorine atom is more electronegative in the following ?
ACH3−ClBCH3−CH2−ClC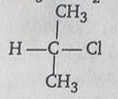 D
D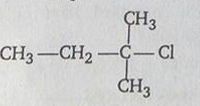
Which chlorine atom is more electronegative in the following ?
ACH3−ClBCH3−CH2−ClC D
D
Which chlorine atom is more electronegative in the following ?
View Solutionwhich chlorine atom is more electronegative in the following ?
View SolutionFluorine has lower electron affinity than chlorine because of
View SolutionWhich chlorine atom is more electronegative in the following ?
View SolutionFluorine is more electronegativity than nitrogen. The best explanation is that:
View Solution

