The correct figure representing isothermal and adiabatic expansions of an ideal gas from a particular initial state is :
A

B
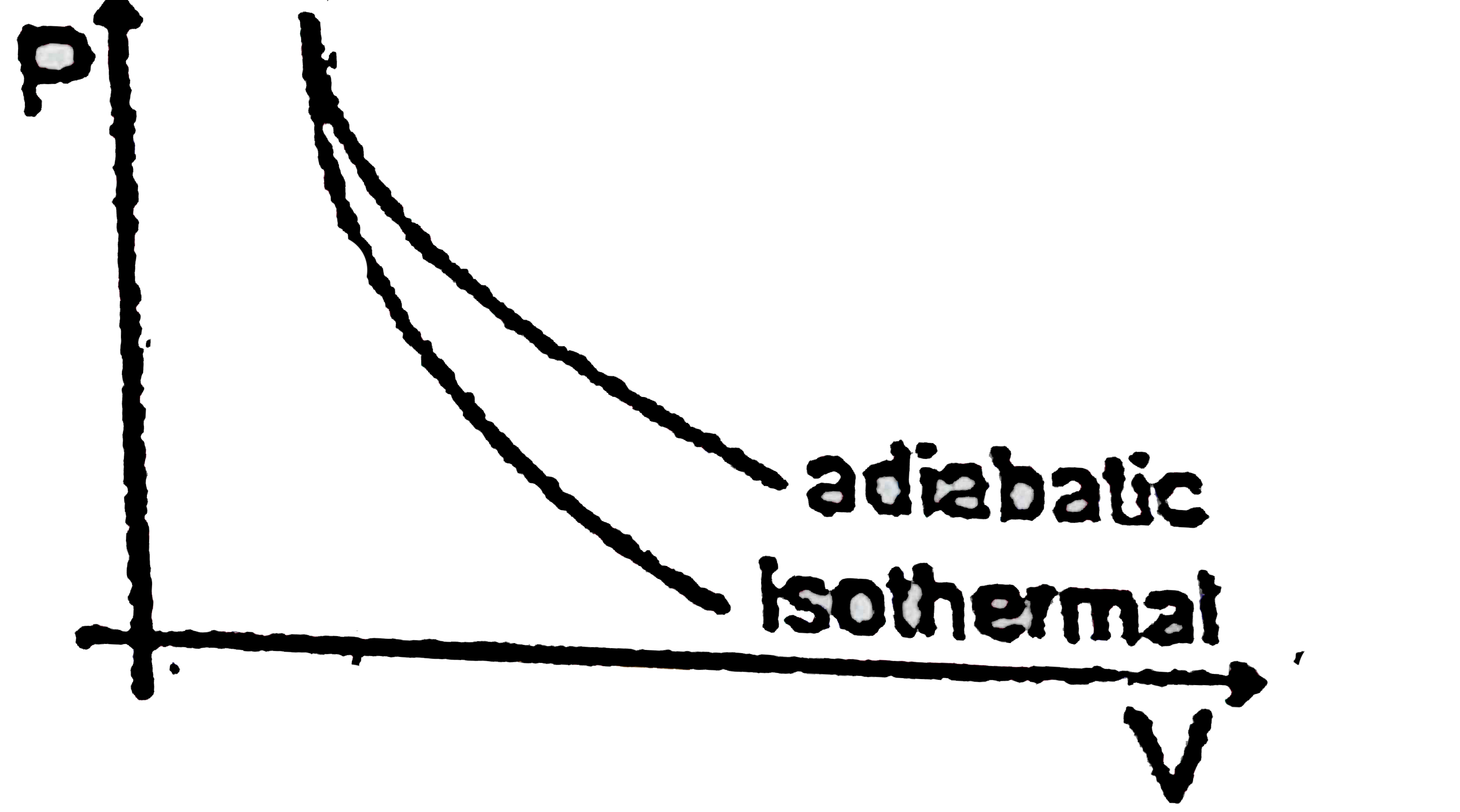
C
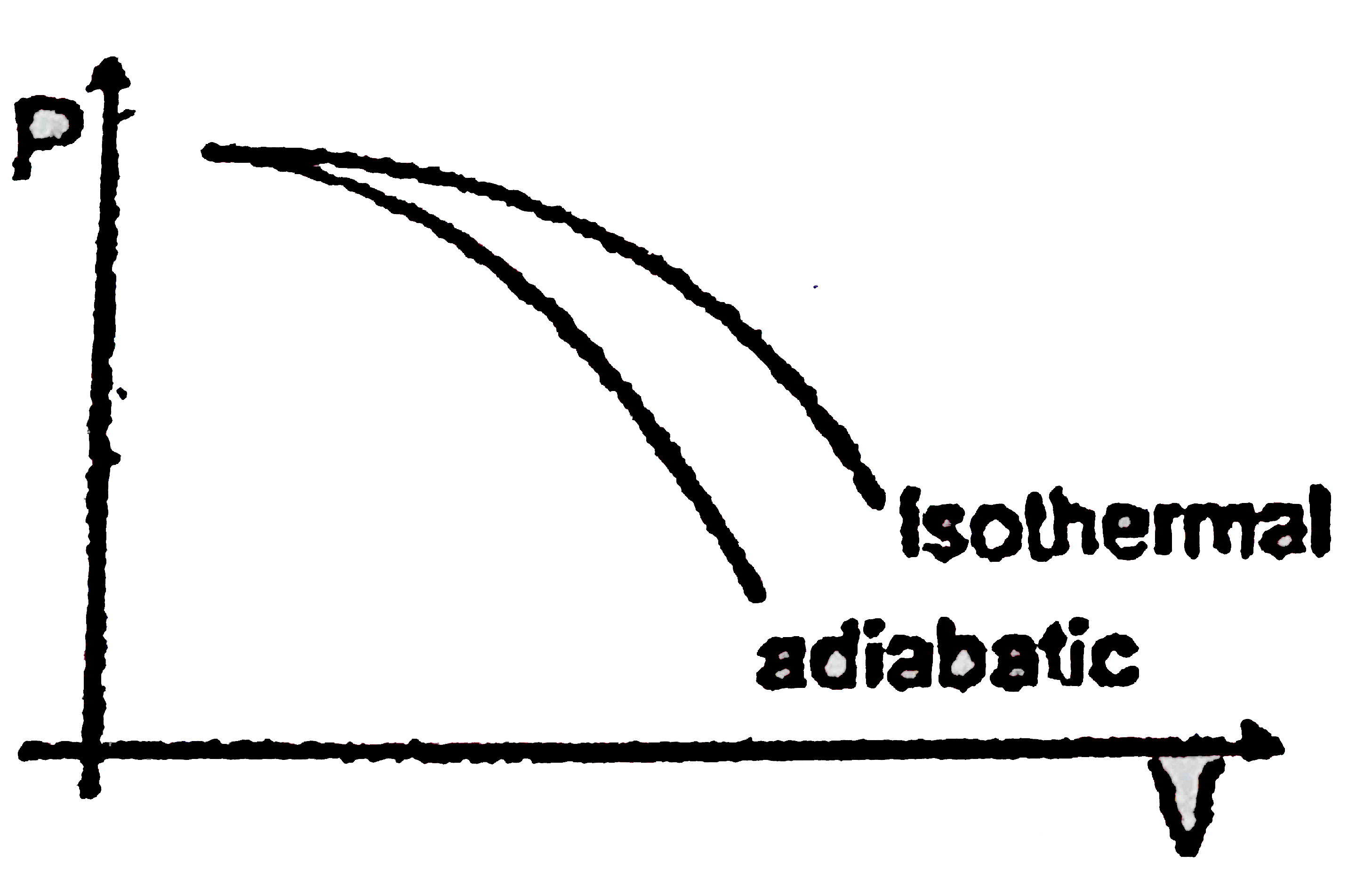
D

Video Solution
Text Solution
The correct Answer is:1
Stops of adiabatic curve is greater than isothermal curve.
|
Similar Questions
Knowledge Check
The correct figure representing isothermal and adiabatic expansion of an ideal gas from a particular initial state is:
A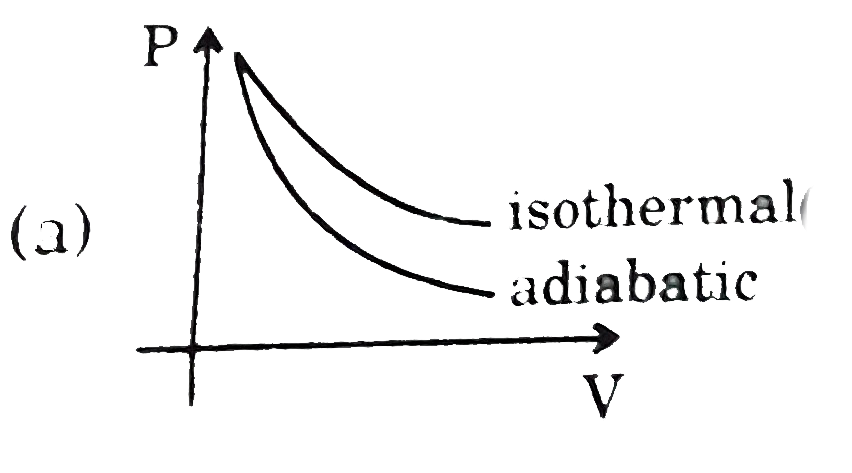 B
B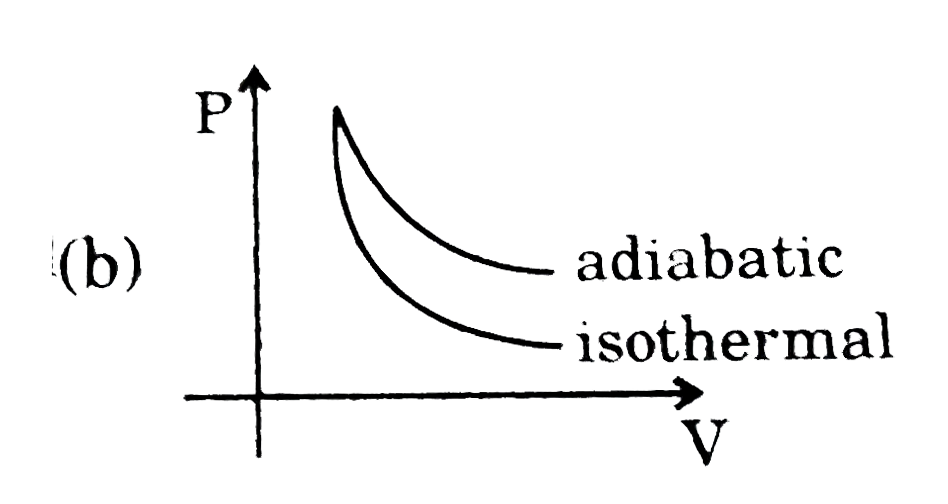 C
C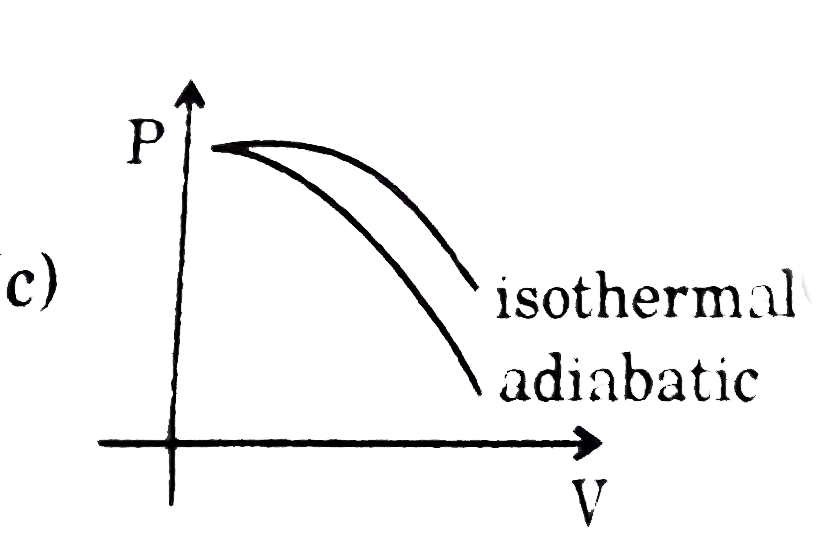 D
D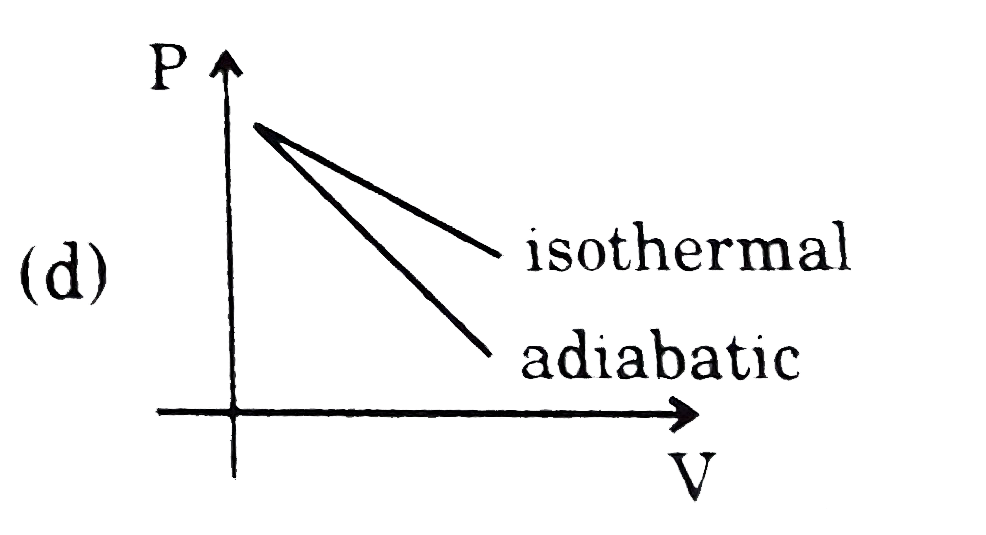
In an adiabatic expansion of an ideal gas -
AW=−ΔEBW=ΔECΔE=0DW=0For the adiabatic expansion of an ideal gas:
APVγ=constantBTVγ−1= constantCTP1−γ= const.DTP1/γ=constant

