What products are expected from the disproportionation reaction of chlorine with hot concentrated solution of sodium hydroxide ?
A
NaCl,NaClO3
B
NaCl,NaClO
C
NaCl,NaClO3
D
NaCl,NaClO2
Video Solution
Text Solution
The correct Answer is:1
6NaOH+3Cl2→5NaCl+NaClO3+3H2O
|
Similar Questions
Knowledge Check
What products are expected from the desproprtionation reactin of hypochorous acid ?
AHCl and Cl2OBHCl and HCIO3CHCIO3 and Cl2ODHCIO2 and HCIO4Which products are expected from the disproportionation reaction of hypochlorous acid?
AHCIO3andCI2OBHCIO2andHCIO4CHCIandCI2ODHCIandHCIO3What product (s) are expected from the following reaction ?
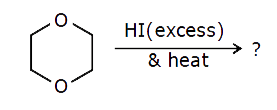 A2ICH2CH2IB2ICH2CH2OHCCH2CH2DCH3CH2I+CH3CH2OH
A2ICH2CH2IB2ICH2CH2OHCCH2CH2DCH3CH2I+CH3CH2OH
Chlorine on reaction with hot and concentrated sodium hydroxide gives :
View SolutionWhat is the major product expected from the following reaction?
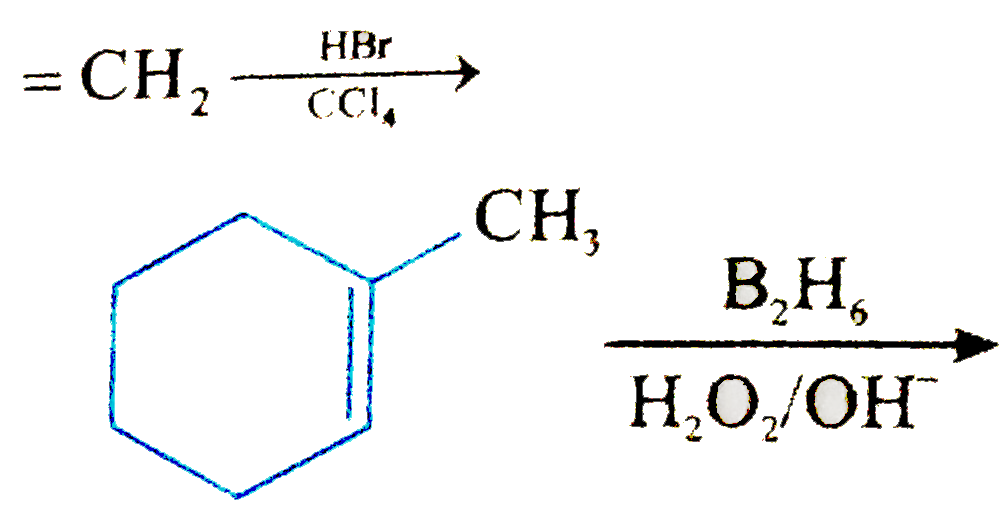
View SolutionWhat is the major product expected from the following reaction?
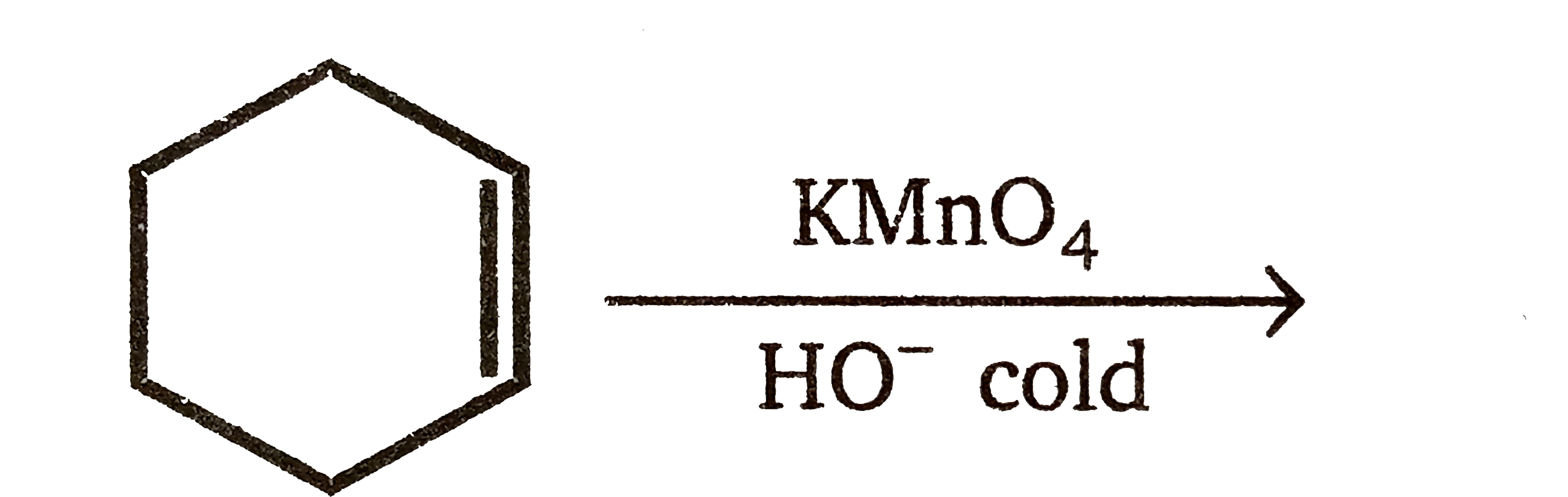
View SolutionWhat is the major product expected from the following reaction?
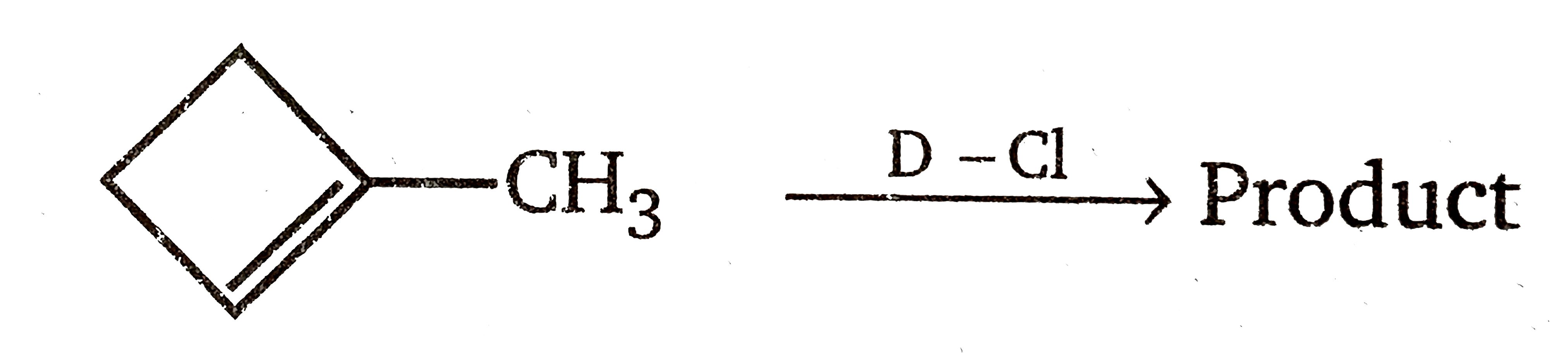
View SolutionChlorine on reaction with hot and concentrated sodium hydroxide gives:
View Solution

