Depression in f.p. of an aqueous urea solutio is 1.86∘.(Kf=1.86∘mol−1kg) Hence, mole− fraction of urea in this soluiton is :
A
0.982
B
0.567
C
0.943
D
0.018
Video Solution
Text Solution
The correct Answer is:4
As ΔTf= molality ×Kf
1.86=m×1.86
molality =1molkg−1
∴ urea =1mol
moles of water =100018=55.55
mole fraction of urea =155.55=0.018
|
Similar Questions
Knowledge Check
Elevation in b.p. of an aqueous urea solution is 0.52∘,(Kb=0.622∘mol−1kg) Hence, mole-fraction of urea in this solution is :
A0.982B0.567C0.943D0.018Figure explains elevation in boiling point when a non-volatile solute is added to a solvent. Variation of vapour pressure with temperaure and elevation in boiling point is marked.
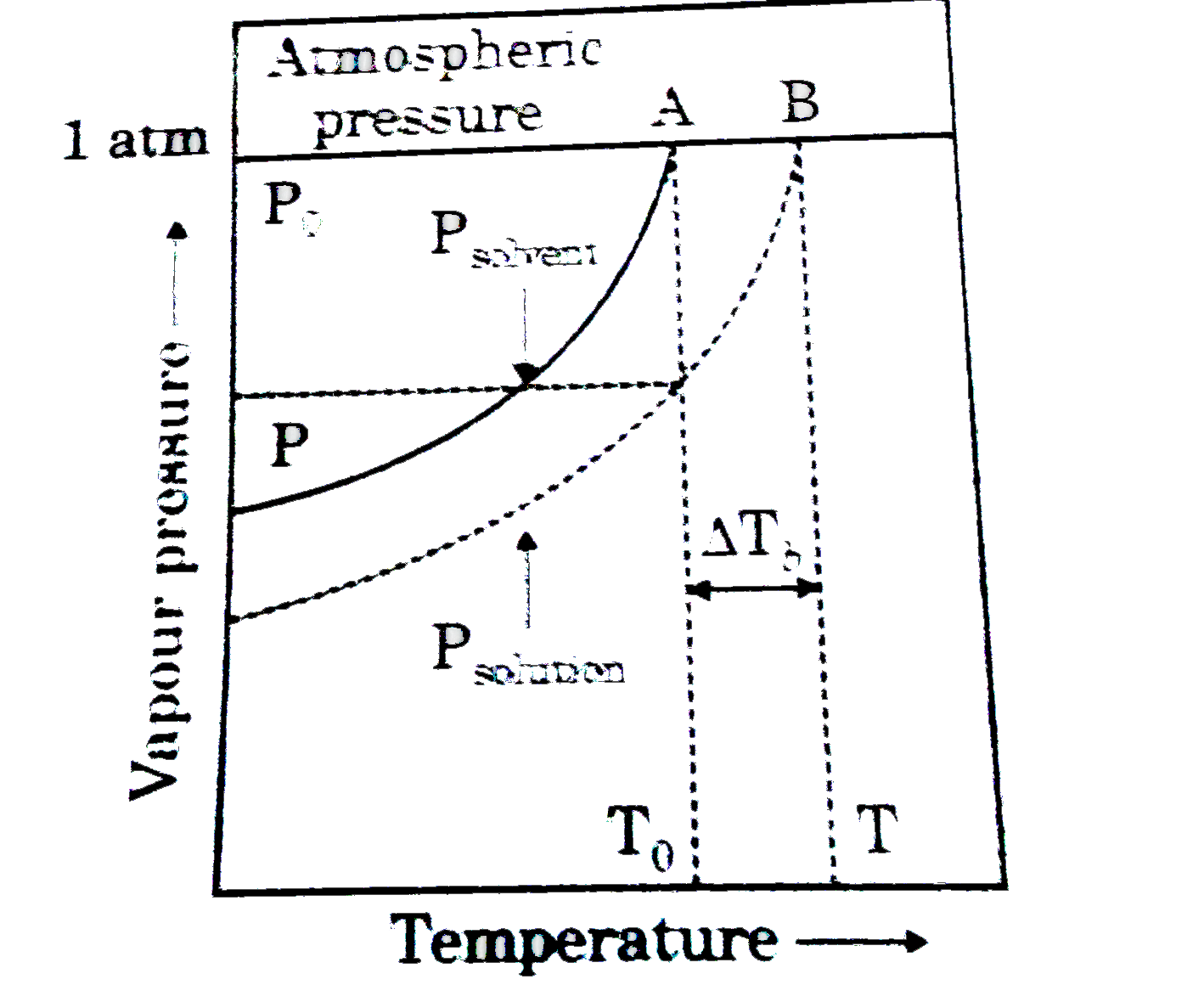
Elevation in b.p of an aqueous urea solution is 0.52∘(Kb=0.52∘mol−1kg). Hence, mole fraction of urea in this solution is:A0.982B0.0567C0.943D0.018Elevation in boiling point of an aqueous solution of urea is 0.52(kbfor water=0.52Kmolality−1). The mole fraction is urea in this solution is :
A0.98B0.0567C0.943D0.02
Mole fraction of a non-electrolyte in aqueous solution is 0.07 . If Kf(H2O) is 1.86∘mol−1 kg , depression in f.p ., ΔTf is :
View Solution0.05 m urea solution will have freezing point (Kf = 1.86 K kg mol−1 )
View SolutionDepression in freezing point is 6K for NaCl solution. If Kf for water is 1.86K kg mol−1, amount of NaCl dissolved in 1kg water is :
View SolutionElevation in boiling point of an aqueous solution of a non-electroluyte solute is 2.01∘ . What is the depression in freezing point of the solution ?
Kb(H2O)=0.52∘mol−1kg
Kf(H2O)=1.86∘mol−1kg
View SolutionWhat is the b.p of an aquoues solution whose freezing point is −2.48∘C ?
(Kf=1.86∘Ckg/mol,Kb=0.512∘C.kg/mol)
View Solution
