
The π electron cloud of C1−C2 is present in the plane of paper than which of the following is incorrect?
Z is perpendicular to the plane of paper
X is present in the plane of paper
σ bond of C2−C3_ is perpendicular to the plane of paper.
π electron cloud of C2−C3 bond and X is present in same plane
The correct Answer is:A, B, C
Only (D) option is correct π electron cloud of C2−C3 is present in same plane.
Similar Questions
Consider the following molecule
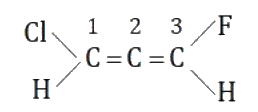
If π− electron cloud of C1−C2 is present in the plane of paper then which of the following is/are correct
View SolutionAccording to VBT any covalent bond will be formed by overlapping of atomic orbitals of bonded atoms provided atomic orbitals must be half-filled and electrons be in opposite spin. According to type of overlapping covalent bonds can be classified as (a) sigma -bond (b) pi -bond (c ) delta -bond : If F_(2)C_(1)=C_(2) " part of " F_(2)C_(1)=C_(2)=C_(3)=C_(4)F_(2) lies in yz-plane, then incorrect statment is :
View SolutionHow many sigma and pi bonds are present in a) C2H2 b) C2H4
View SolutionThe no. of σ & π bonds present in C2H2 respectively are
View SolutionHow many π electrons are present in C2H5 ?
View SolutionHow many pi electrons are present in C_(2)H_(5) ?
View SolutionWhich of the following statements are correct for butadiene
4CH2=3CH−2CH=1CH2
(1) The C1−C2andC3−C4 bonds are longer than a carbon double bond.
(2) The C1−C2andC3−C4 bonds are shorter than a carbon - carbon double bond.
(3) The C2−C3 bond is slightly shorter than a carbon - carbon single bond.
(4) The C2−C3 bond is slightly longer than a carbon - carbon double bond.
View SolutionConsider the following molecule
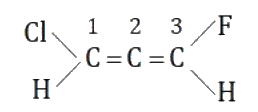
If π− electron cloud of C1−C2 is present in the plane of paper then which of the following is/are correct
View Solution

